![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
19 Cards in this Set
- Front
- Back
|
Describe the subunit composition and the basic structure of prokaryotic 70S and eukaryotic 80S ribosomes including the basis for their names
|
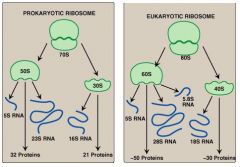
|
|
|
Describe the structure of tRNAs
|
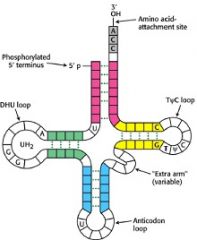
|
|
|
Explaining "charging" a tRNA
|
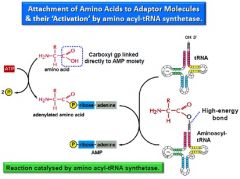
Aminoacylation is the process of adding an aminoacyl group to a compound. It produces tRNA molecules with their CCA 3' ends covalently linked to an amino acid.
Each tRNA is aminoacylated (or charged) with a specific amino acid by an aminoacyl tRNA synthetase. There is normally a single aminoacyl tRNA synthetase for each amino acid, despite the fact that there can be more than one tRNA, and more than one anticodon, for an amino acid. Recognition of the appropriate tRNA by the synthetases is not mediated solely by the anticodon, and the acceptor stem often plays a prominent role. Reaction: 1.amino acid + ATP → aminoacyl-AMP + PPi 2.aminoacyl-AMP + tRNA → aminoacyl-tRNA + AMP |
|
|
Discuss the wobble hypothesis
|
The ‘wobble’ effect allows the 48 different human anticodons to base pair with 61 codons by allowing multiple amino acids to fill the third space.
|
|
|
Describe the sequence of events that occurs during activation in prokaryotes.
|
Activation
Energy is stored in an amino acid by phosporylation and Amino Acyl-tRNA Synthetase then charges a tRNA by esterizing the amino acid to it. |
|
|
Describe the sequence of events that occurs during initiation in prokaryotes.
|
Initiation
Methionine binds to initiator tRNA and then transformylase formylates it into formyl-methionine. The 30S ribosomal subunit and initiation factors form a IF2 (GTP) – Met-tRNA – IF1, + RNA) complex based around the tRNA molecule. When the 50S subunit links, IF1, IF2 and GDP are replaced by it. |
|
|
Describe the sequence of events that occurs during elongation in prokaryotes.
|
Elongation
With the initiator tRNA in the P-site, an aminoacyl tRNA is delivered to the A site by EF-Tu. Peptidyl transferase forms the first peptide bond, linking the new amino acid to Met. EF-G, with a GTP molecule which undergoes hydrolysis, drives translocation - the uncharged tRNA is released and a new codon exposed at the A site as the ribosome move 5’ to 3’ shifting everyone down one notch – A to P to E. |
|
|
Describe the sequence of events that occurs during termination in prokaryotes.
|
Termination
Upon encountering a stop codon, release factor enters the A site triggering peptidyl transferase to hydrolyse the ester bond between the tRNA and the completed polypeptide chain . This releases the protein and dissosciates the ribosome. |
|
|
Describe the A site, P site and E site on the ribosome.
|
A site: the "Acceptor" site where a tRNA lands when entering the ribosome. If a release factor lands here, translation ends.
P site: "Processing" site. The entire ribosome is formed around a tRNA IN this site at the beginning of translation. It is here that the new AA is added to the chain. E site: "Empty" site. Once its AA has been added, a tRNA moves here prior to being booted out of the ribosome. |
|
|
List the major differences between prokaryotic and eukaryotic translation
|
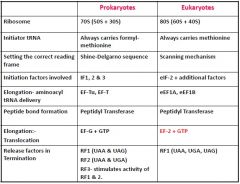
|
|
|
Explain how diptheria toxin interferes with eukaryotic translation
|
Catalyses the transfer of ADP-ribose to host cells EF-2, inactivating it (ribosome cannot translocate) Inhibits protein synthesis
Reaction can be reversed by high concentrations of nicotinamide (overcoming competitive inhibition.) |
|
|
Describe proteolytic processing using insulin as an example.
|
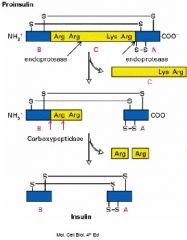
The initial precursor (preproinsulin) contains an amino-terminal signal sequence that targets the polypeptide chain to the ER. Removal of the signal sequence during transfer to the ER yields a second precursor, called proinsulin which is then converted to insulin, which consists of two chains held together by disulfide bonds, by proteolytic removal of an internal peptide (C-peptide).
|
|
|
Review Zymogen activation
|

Expression of Enteropeptidasein the small intestinal
lumen ensures that proteases are onlyactivated at this site & notin the pancreas. Prevents the cleavage of pancreas & pancreatic duct proteins leading to autocatalytic pancreatitis. |
|
|
Review Serine/Threonine phosphorylation
|
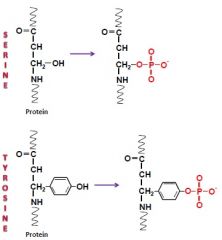
Regulation of enzymes in metabolism.
General Reaction: Transfer of a phosphate group from ATP to an amino acid side chain. Carried out by Kinases, reversed by phosphatases. Most common post-translational modification in eukaryotes – occuring on the OH groups. Can be permanent or reversible. Serine/Threonine is one class of kinases, Tyrosine is another. |
|
|
O-Linked Glycolosation
|
O-linked glycosylation:-
Carbohydrate chains are attached to Serine & Threonine OH groups. Occurs only after the protein reaches the Glogi Apparatus |
|
|
Review O-linked phosphorylation
|
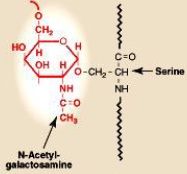
O-linked glycosylation: Carbohydrate chains are attached to Serine & Threonine OH groups. Occurs only after the protein reaches the Glogi Apparatus
Catalysed by Glycosyltransferases |
|
|
Review N-linked phosphorylation
|
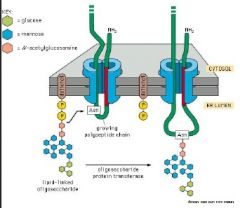
N-linked glycosylation: Carbohydrate chains are attached to the amide nitrogen of Asparagine. N-linked glycosylation is initiated with a lipid linked intermediate DOLICHOL PHOSPHATE at the cytoplasmicsurface of the ER.
Oligosaccharide is transferred from its dolichol carrier to an asparagine residue of the polypeptide as it emerges into the ER lumen. N-linked glycosylation = co-translational. Facilitates correct protein folding. Catalysed by Glycosyltransferases. |
|
|
Lipid Anchoring
|
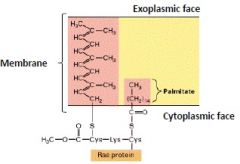
Farnesyl groups to RAS
Certain proteins are anchored to the plasma membrane by either a particular 15-carbon or a 20-carbon group of atoms, called farnesyl and geranylgeranyl groups, respectively. The Cytosolic protein RAS is anchored to the cyctosolic face of the membrane through farnesyl & palmitoyl groups. |
|
|
What is accomplished through Glycolysation?
|
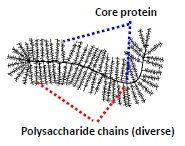
Glycosylation alters the properties of proteins, changing their stability, solubility & physical bulk.
The carbohydrate moieties act as recognition signals that:- Direct protein targeting to either the plasma membrane or to organelle interiors or organelle membranes. Influence cell-to cell interactions. Involved in the development of an organism. Formation of Extracellular Matrix Proteoglycans Modulation of immune response Contributes to differentiation events in organism development |

