![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
8 Cards in this Set
- Front
- Back
|
With regards to percent concnetrations what is commonly identified?
|
Weight Percent (w/w) - towo solids in solution , g solute/100gsolution
Volume percent (v/v) - two liquids in solution, mL solute/100mL solution Weight/volume percent (w/v) - most common % concentration involving a solid dissolved in liquid solution, g solute/100mL solution |
|
|
How is a unit of a compound identified?
|
It is identified with the mole which is measured in grams of a particular atom. The mole is used as a standard measure for comparing substances/drugs
|
|
|
What is Molarity?
|
Measure of moles of solute/L of solution. In order to make this you would weigh out in grams the number of moles you wanted and put into that amount of solution. (Simple I know... They had to make it so confusing!)
|
|
|
With regards to percent concnetrations what is commonly identified?
|
Weight Percent (w/w) - towo solids in solution , g solute/100gsolution
Volume percent (v/v) - two liquids in solution, mL solute/100mL solution Weight/volume percent (w/v) - most common % concentration involving a solid dissolved in liquid solution, g solute/100mL solution |
|
|
How is a unit of a compound identified?
|
It is identified with the mole which is measured in grams of a particular atom. The mole is used as a standard measure for comparing substances/drugs
|
|
|
What is Molarity?
|
Measure of moles of solute/L of solution. In order to make this you would weigh out in grams the number of moles you wanted and put into that amount of solution. (Simple I know... They had to make it so confusing!)
|
|
|
Drug A more potent
Both drugs have same efficacy |
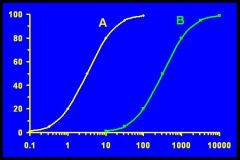
Which drug is more potent?
Which drug is more efficacious? |
|
|
Which parameter tells you the final concentration of drug in various compartments and which one tells you only the rate of transfer between the compartments? Your choices are pKa, pKb, and pKp
|
The Ka and Kb tell you the final concentration of equilibrium between two compartments while Kp the lipid solubility ratio will tell you how rapidly it reaches those values (I think).
|

