![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
47 Cards in this Set
- Front
- Back
|
white blood cells are also know as ...
|
leukocytes
|
|
|
what are the 3 most abundant leukocytes (in order of abundance):
1. 2. 3. |
1. neutorphil
2. lymphocyte 3. monocyte |
|
|
what type of leukocytes are derived from a common myeloid precursor in the bone marrow:
1. 2. 3. 4. 5. 6. |
1. neutorphils
2. eosinophils 3. basophils 4. monocytes 5. dendritic cells 6. mast cells |
|
|
what type of leukocytes are derived from a lymphoid precursor:
1. 2. |
1. B and T cells
2. natural killer cells |
|
|
what type of blood cells are derived from an erythoid precrusor:
1. 2. |
1. platelets
2. erythrocytes |
|
|
what are macrophages:
|
monocytes that have moved out of the bloodstream into tissue
|
|
|
... are usually the first effector cells/phagocytes that a pathogen encounters when it invades the body
|
Macrophages
|
|
|
Macrophages are (long/short) -lived and participate in (innate/adaptive/both) immunity
|
long
both |
|
|
Macrophages are activated upon binding of specific ... to several types of cell-surface receptors. Activation leads to enhanced ... and release of ...
|
ligands
phagocytosis inflammatory cytokines |
|
|
macrophages and other phagocytes utilize ... that recognize conserved molecular moieties called ... that are frequently found on bacteria, viruses, and fungi
|
pattern recognition receptors (PRR)
pathogen-associated molecular patterns (PAMPs) |
|
|
binding of ... ligands to ... by ... induce intracellular signaling in the phagocyte, leading to enhanced phagocytosis and cytokine secretion
|
PAMP
PRRs macrophages and other phagocytes |
|
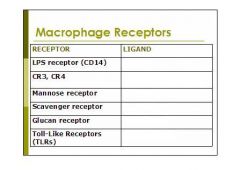
fill in the table:
|
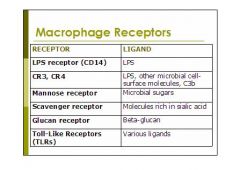
(see figure)
|
|
|
what are the macrophage receptors discussed in class:
1. 2. 3. 4. 5. 6. |
1. LPS receptor (CD14)
2. CR3, CR4 3. Mannose receptor 4. Scavenger receptor 5. Glucan receptor 6. Toll-Like Receptors (TLRs) |
|
|
toll-like receptors are 10 receptors with specificities for ... and the activation of TLRs results in increased production and secretion of ... by ...
|
different microbial products
cytokines macrophages |
|
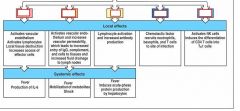
identify the important cytokines
|
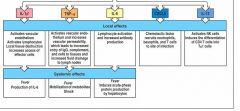
(see figure)
|
|
|
..., ..., and ... are referred to as endogenous pyrogens because they result in heat production in the body and act on temperature-control sites in the ..., and on ... and ..., altering energy mobilization to generate heat.
|
IL-1
IL-6 TNF-alpha hypothalamus muscle fat cells |
|
|
what causes fever:
1. 2. |
1. hypothalamic response to cytokines
2. muscle and adipose cells alter energy mobilization to generate heat |
|
|
what are the effects of fever:
1. 2. 3. 4. |
1. bacterial and viral replication decreased
2. antigen processing is enhanced 3. adaptive immunity more potent 4. human cells become more resistant to negative effects of TNF-alpha |
|
|
In addition to causing fever, IL-1, IL-6, and TNF-alpha act on ...(cell type) in the ... to induce synthesis of acute-phase proteins. These acute-phase proteins include ... and ... both of which act as opsonins and complement activator, and ... a protein involved in blood clotting.
|
hepatocytes
liver C-reactive protein mannose-binding lectin fibrinogen |
|
|
why is mannose-binding lectin highly specific for only bacterial cells:
|
due to the proper spacing of mannose and fucose residues on the bacterial cell surface
|
|
|
C-reactive protein and mannose-binding lectin both act as ... and ...
|
opsonins
complement activators |
|
|
what are the 5 main facts about neutrophils discussed in class:
1. 2. 3. 4. 5. |
1. recruited to sites of infection by macrophage inflammatory mediators (esp. CXCL8)
2. NOT found in normal healthy tissue 3. most abundant circulating WBC 4. short-lived (less than 2 days) 5. about 60% of hematopoietic activity in bone marrow devoted to neutrophil production |
|
|
the primary function of neutrophils is ... and they form ... upon death
|
powerful phagocyte
pus |
|
|
During phagocytosis, there is an increase in ... and ... consumption which is referred to as the ... which allows for ... killing of bacteria through the generation of reactive oxygen species. In addition, bacteria can be killed by pre-formed substances released from ... or ... when they fuse with the phagosome. This is referred to as ... killing.
|
glucose
oxygen respiratory burst oxygen-dependent granules lysosomes oxygen-independent |
|
|
Chronic Granulomatous Disease:
1. caused by: 2. what gets impaired: 3. what is the result: 4. and what is also the result: |
1. inherited deficiency in NADPH oxidase
2. phagocytic oxygen-dependent killing 3. chronic, recurrent infections with catalase-positive microorganisms (Staphylococcus, Klebsiella, Serratia, Aspergillus) 4. chronic inflammatory symptoms like gingivitis, enlarged lymph glands, tumor-like granuloma masses |
|
|
Neutrophils are usually the (first/second) effector cell encountered by a pathogen. because they must migrate from out of the ... into the ... in response to ... cytokine and chemokine release.
|
second
bloodstream infected tissue macrophage |
|
|
how do neutrophils migrate from blood into infected tissue:
|
they roll along vascular endothelial surface becuase selectin-mediated adhesion to s-Lex is weak
|
|
|
what are the 4 steps in neutrophil extravasation:
1. 2. 3. 4. |
1. Weak rolling adhesion of the neutrophil along the endothelial wall mediated by E-selectin
2. Tight binding between the neutrophil and endothelial cell that traps neutrophil - mediated by ICAM-1 3. Diapedesis involves the ameoboid-like movement of the neutrophil in between two adjacent endothelial cells 4. Migration of the neutrophil through the interstitial fluid to the site of infection - mediated by CXCL8 |
|
|
important things to remember about the adhesion molecules selectins do:
1. 2. 3. 4. |
1. stored in Weibel-Palade bodies inside vascular endothelial cells
2. expressed at cell surface in response to inflammatory mediators 3. weakly bind to sialyl-Lewisx carbohydrates of cell-surface glycoproteins on leukocytes 4. allow for neutrophil “rolling” |
|
|
adhesion molecules to remember (and why): integrins:
1. 2. 3. 4. |
1. LFA-1 and CR3 on neutorphil cell surface
2. ICAM-1 on endothelial cells 3. CXCL8 stops neutorphil rolling 4. LFA-1 and CR3 in diapedesis |
|
|
1. what casues Leukocyte Adhesion Deficiency:
2. what effect does it have on leukocytes: 3. what does not get formed: 4. what are the symptoms: |
1. absence of CD18 – the common β2 chain of many integrin molecules
2. unable to undergo adhesion-dependent migration 3. abscess or pus 4. recurrent, chronic bacterial infections (omphalitis common in infants) |
|
|
NK cells are the effector cells that kill .... and ... NKs are very important in combating ... infections (along with interferons).
Killing is accomplished through the release of ... and ... that form pores in the target cell membrane, leading to the induction of apoptosis. |
infected cells in the body
pre-cancerous and cancer cells viral granular perforins granzymes |
|
|
cytotoxicity of NKs is increased dramatically by ..., ..., and ...
|
IFNs
IL-12 TNF-alpha |
|
|
..., ..., and ... increase the proliferation and activation of NK cells to quickly eliminate viral infections
|
IFNs
IL-12 TNF-alpha |
|
|
NK cells express a variety of receptors that help them recognize ... present on the surface of infected cells. NK receptors can either be ...-like or ...-like in structure. In addition, NK receptors are either ... or ... in function.
|
ligands
immunoglobulin lectin activating inhibitory |
|
|
NK cells have ... and ... receptors that interact with ligands, Upon interaction with a ... of a healthy cell, the inhibitory signal always dominates, preventing activation of the NK cell
|
activating
inhibitory MHC class I molecules |
|
|
what happens to MHC class I molecules of infected cells:
|
its conformation may change or its expression may be lost
|
|
|
alloreactions are mediated by ...
|
NKs
|
|
|
what are the important facts about eosinophils:
1. 2. 3. 4. |
1. second most abundant granulocyte
2. defend against helminth worms and other intestinal parasites 3. involved in allergies and asthma 4. release cytokines, lipid mediators, and reactive oxygen species through degranulation |
|
|
what are the important facts about basophils:
1. 2. 3. |
1. least abundant granulocyte
2. degranulate releasing histamine and other powerful cytokines, as well as proteolytic enzymes and other inflammatory mediators 3. allergic responses |
|
|
what are the minority subpopulations of B and T cells that contribute to innate immunity:
1. 2. they are considered to be a component of the innate immune response due to their ... |
1. B-1 lineage of B cells
2. γ:δ T cells presence at birth |
|
|
what are the Major sequence of events in the innate immune response:
1. 2. 3. |
1. Complement activation
2. Macrophage activation 3. Neutrophil extravasation and activation |
|
|
Recruitment of neutrophils into tissue, peaks within ...hours
Neutrophil-derived chemoattractants recruit more monocytes, macrophages, and eosinophils within ... hours |
6
5 to 6 |
|
|
what are the major players of the innate immune response:
1. 2. 3. 4. 5. |
1. Complement
2. Macrophages 3. Neutrophils 4. Chemokines and cytokines 5. NK cells |
|
|
what are the hallmarks of inflammation:
1. 2. 3. 4. |
1. calor (heat)
2. dolar (pain) 3. rubor (redness) 4. tumor (swelling) |
|
|
Phagocytes like ... and ... become “antigen-presenting cells” that activate .... Cytokines released during the innate immune response “prime” the adaptive immune response.
|
macrophages
dendritic cells T cells |
|
|
Inflammation results from vascular changes that occur in local blood vessels in response to cytokines: (1) ... and (2) ...
|
1. Blood flow increases to the site of infection (to get more effector cells there more quickly)
2. vascular permeability increases (spaces between adjacent endothelial cells widen to allow for easy extravasation of phagocytes out into infected tissue) |

