![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
90 Cards in this Set
- Front
- Back
|
What is the purpose of a diagnostic test and to whom is it applied? |
Purpose is to confirm a disease and establish a diagnosis applied to patients in whom there is clinical suspicion of disease = high pre-test probability of disease |
|
|
What is a screening test's purpose and to whom is it applied? |
Purpose is to identify patients who MAY have disease Applied to people in whom there is no clinical suspicion of disease Results are preliminary and want diagnostic tests |
|
|
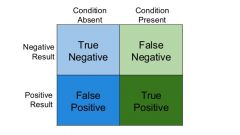
In diagnostic tests, what are true and false negatives and true and false positive? |
True negative = tested negative, negative for disease True positive = tested positive, and positive for disease False negative = tested negative, and positive for disease False positive = tested positive and negative for disease |
|
|
What is the sensitivity of a diagnostic test and how is it calculated? what is the specificity of a diagnostic test and how is it calculated? |
Sensitivity = % of people WITH disease that test positive =T. pos/(T.pos +F.neg) Specificity = % of people WITHOUT disease that test negative = T.neg/(T.neg +F.pos) |
|
|
What is a positive predictive value? A negative predictive value? |
Positive predictive value= Proportion of positive tests that are indeed positive for disease = TP/(TP+FP) Negative predictive value = proportion of negative tests that are truly negative = TN / (TN+FN) |
|
|
Explain how the prevalence of disease in your test population affects the PPV and NPV values for the test (but not the sensitivity and specificity |
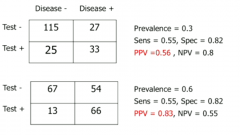
If you sample a population with very low prevalence, the majority of positives will be false positives |
|
|
How is PPV related to underlying prevalence of disease? and NPV |
PPV is positively correlated with high prevalence. NPV negatively correlated. |
|
|
Give an example of how finding a NPV or PPV can tell you nothing useful in a population of a certain prevalence. |
If the prevalence is tiny, then of course you'll have a high NPV. If prevalence is 0, then NPV HAS to be 100% If prevalence is 100%, then of course PPV will be high. Not very informative |
|
|
What is a likelihood ratio for a diagnostic test? How do we calculate the LR for positive and negative tests? |
It's the likelihood that a given test result would be expected in a patient with the disease compared to a patient without the disease. LR of positive result = sensitivity / (1-specificity) tells you how much the odds of the disease increase when a test is positive. LR of negative result = (1-sensitivity)/ specificity tells you how much the odds of the disease decrease when a test is negative. |
|

Break down the logic of the likelihood ratio normogram. |
Normograms use premade variable scales to convert a pretest probability (%) into a post-test probability via a likelihood ratio. Basically post-test probability is a fixed function of both LR and pre-test probability and the normogram is a rough and ready way to visualize it. |
|
|
How does changing the threshold for defining a disease state (e.g. BP or FEV1) affect sensitivity and specificity? |
If you lower the threshold, you will improve sensitivity but lower specificity If you raise it, you will improve specificity but lower sensitivity |
|
|
Distributions demonstrating how changing thresholds will change specificity/sensitivity. |

|
|
|
What is a receiver operator characteristic (ROC) curve and why is it useful? |
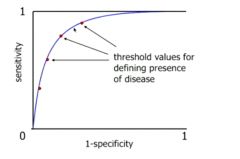
It is a plot of (1-specificity) against sensitivity for various thresholds for a diagnostic test It is a graphical representation of the trade-off between sensitivity and specificity in tests - we want a reasonable trade off |
|
|
Where does an ideal test sit on the ROC curve? Its it realizable? How do we measure the discriminating ability of a test in an ROC curve? |
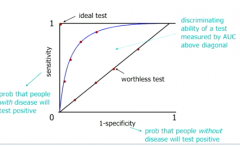
Ideal test = 100% selecitivty and specificity. Not possible because every test will throw out false results SOMETIMES. We measure the ability of test to discriminate by area between curve and the worthless diagonal. |
|
|
Puzzle over the ROC curve |
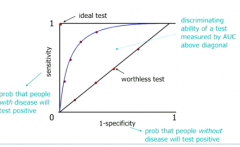
|
|
|
What does it mean when sensitivity and (1-specificity) are equal at all thresholds? |
The test has no discriminatory power; it is just as likely to pick up people with disease as people without |
|
|
What is a roughly high likelihood ratio? |
Greater than 10 |
|
|
What three findings give a very high LR for COPD? |
Wheeze Self-reported history of COPD FET > 9 sec |
|
|
What are screening tests used to identify in a population? |
1. Risk factors present ? (primary prevention 2. Early disease present? (secondary prevention) |
|
|
What are some of the WHO criteria for introducing a screening test for the wider population? |
• important health problem • natural history well understood • detectable early stage • early treatment beneficial • suitable test for early disease • adequate healthcare provision for extra workload • risks (including psychological) less than benefits |
|
|
What are some biases in the measurement of screening effectiveness? |
Selection: healthy more likely to self-select to come along to screening lead-time bias: you're just detecting cancer earlier, but you're not actually improving the outcomes Length-time - you're more likely to detect non-aggressive diseases |
|
|
Explain why lead-time bias makes it seem as though screening increases survival time when it's not really the case? |
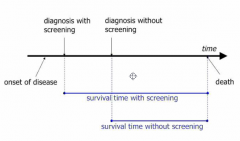
In certain cases, you haven't lengthened the paitent's lifespan, you've simply started the clock earlier. |
|
|
What is overdiagnosis bias in screening? |
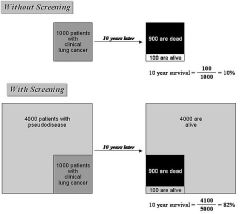
Screening programs can detect cases of cancer that would not lead to death in individual's lifetime and would not have been detected otherwise. Screening skews to pick up indolent cancers that will never cause problems. We'd never have known about it otherwise. |
|
|
What are two strategies for screening? |
Population based: Test is offered systematically to all individuals in target group. Opportunistic: test offered to an asymptomatic individual when they present to doctor for unrelated reasons. |
|
|
What is pre-test probability and post test probability? |
Pre-test probability: the proportion of people in the population at risk who have the disease at a specific time or time interval, i.e. the point prevalence or the period prevalence of the disease. Post-test probability: the proportion of patients testing positive who truly have the disease |
|
|
What are the divisions of the bronchial tree, from largest to smallest? |
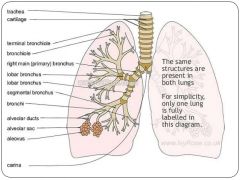
Trachea Main bronchus Lobar bronchus Segmental bronchus Bronchiole Terminal bronchiole Respiratory bronchiole Alveolar ducts, sacs and alveoli = acinus |
|
|
What cell types form the bronchi/bronchioles? |
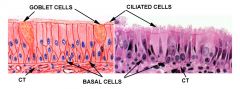
1. respiratory epithelial cells (columnar, pseudostratified and ciliated) 2. Goblet cells 3. Neuroendocrine cells 4.Basal cells (respond to injury by migrating upwards) |
|
|
What is the difference between obstructive and restrictive lung disease? Give examples of each (MAJOR) |
Obstructive = reduction in airflow Restrictive = reduction in lung volume/expansion Obstructive: Ashtma Bronchiectasis COPD = chronic bronchitis, emphysema (most patients have both at same time) Restrictive: pulmonary fibrosis Pneomoconiosis Sarcoidosis (appearance of sarcoid granulomas) |
|
|
What is emphysema? |
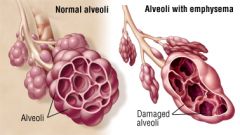
Emphysema is characterized by irreversible enlargement of the airspaces distal to the terminal bronchiole, accompanied by DESTRUCTION of their walls without obvious fibrosis |
|
|
How does emphysema cause airway obstruction? |
LOSS OF ELASTIC RECOIL Narrowing of the airways occurs due to inflammation and scarring within them. This contributes to the inability to breathe out fully. The greatest reduction in air flow occurs when breathing out, as the pressure in the chest is compressing the airways at this time.[55] This can result in more air from the previous breath remaining within the lungs when the next breath is started, resulting in an increase in the total volume of air in the lungs at any given time, a process called hyperinflation or air trapping |
|
|
What are the four types of emphysema? |
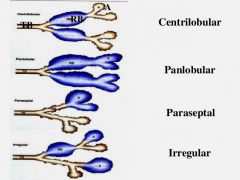
Centriacinar (centrilobular) Panacinar (panlobular) Distal acinar (paraseptal) Irregular |
|
|
Give the pathogenesis of emphysema |
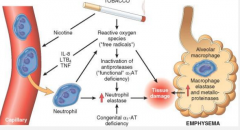
1. Smoking/ air pollution and genetic factor introduce noxious particles into the acini = ROS. 2. Resident epithelial cells and macrophages are affected and release LTB4, IL-8, TNF, which bring in inflammatory cells (chemokines), amplify inflammation (cytokines) and induce structural changes (growth factors) 3. Neutrophils release neutrophil elastase, macrophages release macrophage elastase 4. In genetically compromised indivudal,s there is a lack of antiproteases to stop this. 5. Tissue damage results 6. infection can exacerbate the inflammation. |
|
|
What complications can arise from emphysema? |
Hypoxia caused by airflow obstruction and loss of diffusion capacity Pulmonary hypertension, cor pulmonale Pneumothorax |
|
|
What is bullous, compensatory and interstitial emphysema? |
Bullous = localized accentuations of emphysema form subpleural blebs (bullae) near the apex. Compensatory = dilation of lung alveoli in response to loss of lung substance elsewhere, i.e. in surgery Interstitial = entrance of air into the connetive tissue stroma of lung. Can occur when alveolus tears under high pressure when coughing. |
|
|
What is chronic bronchitis? |
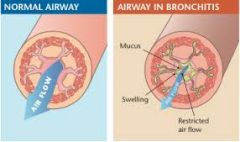
Chronic bronchitis is defined clinically as persistent cough with sputum production for at least 3 months in at least 2 consecutive years, in the absence of any other identifiable cause |
|
|
What is a productive and unproductive cough? |
Productive = contains sputum (mucus) Unproductive = dry and does not contain sputum The reason we cough physiologically is to get rid of microbes, irritants and mucus! |
|
|
Give the pathogenesis of chronic bronchitis? |
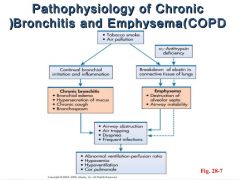
1. Exposure to noxious/irritants like tobacco smoke, dust etc. 2. Damage of airway lining cells/interference with ciliary action of respiratory epithilium 3. Neutrophils, lymphocytes and macrophages emigrate to site in both acute and chronic responses 4. Narrowing of small airways via inflammation and fibrosis 5. Release of histamine and IL-13 lead to mucus hypersecretion in larger airways, via increase in goblet cells and hypertrophy of submucosal glands to protect from smoke. |
|
|
What are the complications caused by chronic bronchitis? (3) |
-Superimposed infective exacerbations #1 -hypoxia, pulmonary hypertension, cor pulmonale -Squamous metaplasia or dysplasia (premalignant) |
|
|
What is small airways disease? |
• Caused by cigarette smoke • Chronic inflammation, fibrosis,obstruction of terminal bronchioles(<2mm) • Important component of COPD |
|
|
What is small airways disease? |
|
|
|
What is COPD? |
Chronic obstructive pulmonary disease? >90% caused by smoking • Emphysema, chronic bronchitis, andsmall airways disease (and somereversible bronchospasm or “asthma”)in varying proportions • Characterised by slow progression withsuperimposed infective exacerbations |
|
|
What can make COPD worse? |
Infective exacerbatiosn are a concern e.g. bacterial bronchitis causing bronchospasm |
|
|
COPD is a spectrum of bronchitis and emphysema mixed together. What are the hallmarks of each in a clinical presentation (table)? |
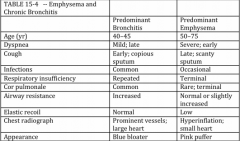
|
|
|
What is the composition of cigarette smoke? |
Particulate and gas phases present. Liquid droplets = nicotine, oxidants, >40 carcinogens Gaseous component: air, CO, volatilised hydrocarbons |
|
|
How does smoking increase the likelihood of pulmonary infection? |
– Inhibition of the muco-ciliary escalator – Increased mucus – Inhibition of leukocyte function – Direct damage to the epithelial layer |
|
|
What is bronchiectasis? |
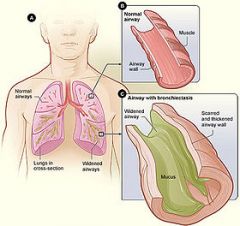
Bronchiectasis is a disorder in which destruction of smooth muscle and elastic tissue by chronic necrotizing infections leads to permanent dilation of bronchi and bronchioles. Because of better control of lung infections, bronchiectasis is now uncommon. irreversible, abnormal bronchial dilation |
|
|
What can cause bronchiectasis? |
Necrotizing infections like staph aureus, aspergillus and influenza Bronchial obstruction clogs off a certain segment of the bronchial tree, where the bronchiectasis occurs Cystic fibrosis |
|
|
Describe the pathogenesis of bronchiectasis. |
Obstruction and infection are the major conditions needed for bronchiectasis. After bronchial obstruction, normal clearing mechanisms are impaired, resulting in pooling of secretions distal to the obstruction and secondary infection and inflammation. Severe infections of the bronchi lead to inflammation, often with necrosis, fibrosis, and eventually dilation of airways. |
|
|
What are the clinical presentations of bronchiectasis? |
Severe, persistent cough (lots of mucus present) foul smelling and bloody sputum dyspnea and orthopnea. |
|
|
What two general conditions fall under restrictive lung disease? |
Chronic inflammation and fibrosis of alveolar interstitium (interalveolar septa) Chest wall disorders |
|
|
What are the clinical presentations of restrictive lung disease? |
Dyspnea End-inspiratory crackles Eventual cyanosis No wheezing |
|
|
What is idiopathic pulmonary fibrosis? |
Idiopathic pulmonary fibrosis (IPF) refers to a clinicopathologic syndrome marked by progressive interstitial pulmonary fibrosis and respiratory failure |
|
|
Give a proposed pathogenesis of IPF (idiopathic pulmonary fibrosis)? |
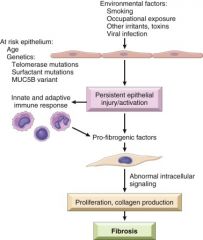
|
|
|
What is usual interstitial pneumonia? |
![Usual interstitial pneumonia (UIP) is a form of lung disease characterized by progressive scarring of both lungs.[1] The scarring (fibrosis) involves the supporting framework (interstitium) of the lung.
It's a HISTOLOGIC PATTERN OF fibrosis.](https://images.cram.com/images/upload-flashcards/79/15/17/24791517_m.jpg)
Usual interstitial pneumonia (UIP) is a form of lung disease characterized by progressive scarring of both lungs.[1] The scarring (fibrosis) involves the supporting framework (interstitium) of the lung. It's a HISTOLOGIC PATTERN OF fibrosis. |
|
|
What is the definition of asthma? |
– increased responsiveness of airways tovarious stimuli leading to episodicbronchoconstriction which is at leastpartly reversible |
|
|
What are the two types of asthma? |
Atopic (evidence of allergen sensitization and immune activation, often in a patient with allergic rhinitis or eczema) non-atopic (no evidence of allergen sensitization) |
|
|
What are some common triggers for asthma? |
Environmental allergens Respiratory tract infection Exercise Cold weather Some drugs Inahled chemicals |
|
|
What are the short term complcations of ashthma? |
Death Atelectasis (lung collapse) Spontaneous pneumothorax or pneumomediastinum |
|
|
What are the complications of long term chronic ashthma? |
Airway remodelling - fibrosis and irreversible obstruction chronic hypoxia --> pulmonary hypertension --> cor pulmonale |
|
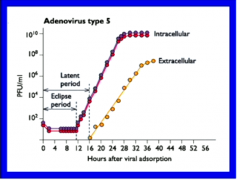
|
PFU/ml =measure of viral presence. We can only measure viral effects on other cells, not viruses themselves. Extracellular = sample medium above cells Intracellular = bust cells open and count viruses |
|
|
What is the eclipse period and the latent period of viral growth? |
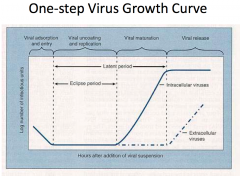
Eclipse period: No phage particles can be detected during this period as the phages are being uncoated and phage DNA is being injected foe replication. Latent period = In the latent period, attachment, entry, replication, transcription, translation and assembly of progeny phages occur. |
|
|
What are the stages of viral replication? |
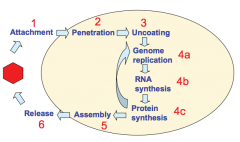
|
|
|
What occurs in viral attachment/adsorption? |
Viral attachment protein binds to a receptor on the plasma membrane of host cell. This interaction limits the host species and cell type that a virus can infect. The virus exploits receptors useful for the cell to get in. |
|
|
What type of receptors do viruses tend to bind to in attachment to host cells? |
Protein (e.g. ICAM-1 for rhinoviruses) Carbohydrate (sialic acid for influenza virus) |
|
|
Describe the process by which HIV virus gains access to CD4+ T-cells. |
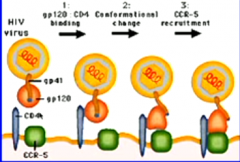
HIV binds to CD4, triggering confirmational change in glycoprotein, allowing the complex to recruit CCR-5. The blue hydrophobic region is outside... |
|
|
What happens in viral penetration of a cell (2 ways in)? |
After adsorption, the coat of enveloped viruses mayFUSE with the host cell membrane and release thevirus nucleocapsid directly into the cytoplasm. • Other viruses (enveloped and non-enveloped) enter thecell by a process of "ENDOCYTOSIS" which involvesinvagination of the cell membrane to form vesicles in thecell cytoplasm. |
|
|
Describe membrane fusion of HIV virus to gain access to host cell. |
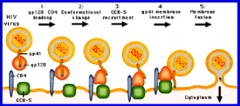
When hydrophobic region on gp41 is forced into polar environment, it plunges into the host cell membrane. This induces membrane fusion via formation of a fusion core. |
|
|
Give an example of a virus that penetrates the host cell via endocytosis and state how. |
Togavirus enters via endocytosis. The virus can get out by producing toxins lytic to the endosome OR by responding to low pHs of endosome through a conformational change that exposes a fusion region. |
|
|
In general where do DNA and RNA viruses replicate? What are the exceptions? |
DNA viruses tend to replicate in the nucleus RNA viruses in the cytoplasm HIV and influenza are RNA viruses that need to replicate in the nucleus Poxvirus is a DNA virus that replicates in the cytoplasm. |
|
|
What proteins are produced in the aerly and late stage of viral genome translation? |
Early proteins are usually non-structural (DNA and RNA polymerases) Late proteins are structural proteins that make up virion/capsid structure. |
|
|
WHY do DNA viruses need to replicate in the nucleus? |
They need DNA-dependent DNA polymerases. In host, these are only present in the nucleus! |
|
|
Describe the genome of poliovirus. |
It is a linear, single stranded plus sense RNA + sense means it is the same sense as mRNA. |
|
|
Describe the replication process of the RNA virus, poliovirus. |
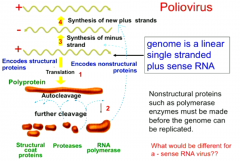
Poliovirus enters cell and, being a +sense RNA, it can be read to make proteins. It makes a polyprotein via translation, which undergoes autocleavage. Cleavage products include RNA-dependent RNA polymerase |
|
|
What problem is apparent in replication for a -ve sense RNA virus? How do they get around this? |
They can't make protein because they can't be read by host machinery
To get around this, they bring RNA polymerase WITH THEM inside the virion so they always have the tools to re-assemble once penetrated |
|
|
What are the baltimore classifications (Baltimore group I, II , III, IV, V, VI)? |
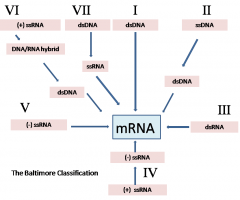
Group 1 is most like us. Group VI are the retroviruses. |
|
|
Which baltimore class viruses NEED to have their polymerase in the virion? |
The Class V viruses. They are negative sense RNA viruses. |
|
|
What steps are involved in viral translation and protein processing? (3) |
• Translation of structural and non-structuralproteins is carried out by ribosomes in thehost cell cytoplasm. • Post-translational cleavage of polyproteins ortrimming of structural proteins usually needsvirus-coded proteases. • Glycosylation of envelope glycoproteinsoccurs in RER and Golgi vesicles whichresults in them being deposited on the cellsurface. |
|
|
Compare the assembly and release strategies of enveloped and non-enveloped viruses. |
Non-enveloped virsuses all have an icosahedral structure. The translated proteins spontaneously assemble around the nucleic acid. Once there are enough virions, the cell has no choice but to burst. Enveloped viruses: Need to use BUDDING |
|
|
What happens in virus budding? |
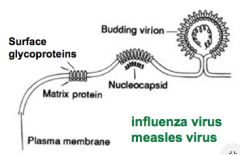
Patches of viralenvelope glycoproteinsaccumulate in theplasma membrane. Capsid proteins andnucleic acid condensedirectly adjacent to thecell membrane The membrane surrounding the nucleocapsid then bulges outand becomes "nipped off" to form the new enveloped virion. |
|
|
What is the secretory pathway of virus release? |
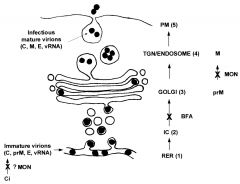
Virusparticles enclosed within Golgi-derivedvesicles are released tothe outside of the cell when thetransport vesicle fuses with thecell membrane. |
|
|
What are the four possible changes to cells that result from viral infection? |
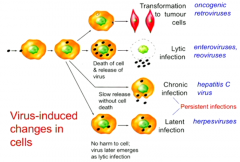
1. Lytic infection 2. Chronic infection (virus replicates in cell without ever killing it) 3. Latent infections (just sit inside the cell for years and years and then emerge) 4. Transformation to tumour cells. |
|
|
What is a cytopathic effect? |
CPE refers to structural changes in host cells that are caused by viral invasion |
|
|
What are inclusion bodies? |
Inclusions represent accumulated viral proteins at the siteof virus assembly Can have nuclear inclusions and cytoplasmic inclusions (DNA and RNA viruses respectively) |
|
|
What are oncogenes in viral replication? |
O,ncogenes code for proteins with growth promoting properties andtheir expression can lead to uncontrolled proliferation of the infected celland tumour development. Other viruses cause tumours because theirreplication affects the cellular version of an oncogene. |
|
|
Which type of virus is more prone to genome mutation? |
RNA viruses - they produce their own RNA polymerases which aren't as good at detecting mistakes. The DNA viruses use our own polymerases, which are pretty good at checking for errors in replication. |
|
|
Describe recombination and reassortment in viral mutation? |
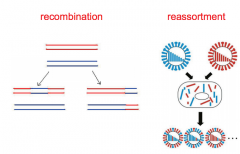
• recombination (exchange of stretches of nucleic acidbetween genomes of similar sequence - esp. DNA viruses) • reassortment (swapping of segments for viruses thathave segmented genomes) |
|
|
What five things can halt viral infection? |
• antibody that blocks uptake and/orneutralises progeny virus • killing the infected cell by cytotoxic Tcells, NK cells or Ab-mediatedmechanisms • interferon ( a specific antiviral cytokine) • blocking the replication cycle by specificantiviral drugs |
|
|
What is acyclovir? |
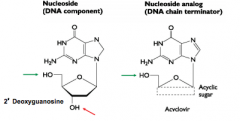
USED AGAINST HERPESVIRUS Acyclovir is a nucleoside analogue, specifically guanosine. Phosphate groups can be added so that the analogue can be incorpated into DNA BUT THE 3' HYDROXY GP required to extent the DNA polymer is ABSENT |
|
|
How does acyclovir take down Herpesvirus? |

Acyclovir is converted into acyclovir triphosphate using thymidine kinase which ONLY THE VIRUS HAS. Won't occur in uninfected cells. Acyclovir triphosphate incorporation into herpesvirus growing DNA strand will terminate DNA replication and kill infected cell. |
|
|
Picture of bronchitis under the scope. |
|

