![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
15 Cards in this Set
- Front
- Back
|
BREAKPOINT CHLORINATION |
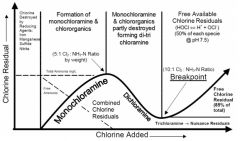
the addition of chlorine to water until the chlorine demand has been satisfied. at this point,further additions of chlorine will result in a free chlorine residual that is directly proportional to the amount of chlorine add beyond the breakpoint. |
|
|
CHLORINE STORAGE |
Chlorinators & storage tanks must be kept above ground in a separate room Temperature: Tanks = above 50°F / 10°C max 100°F / 38°C Tubes = 10°F / 3-6°C above surrounding temperature to prevent condensation |
|
|
TYPES OF CHEMICAL DISINFECTANTS |
1. Chlorine, Cl (chloramines, Cl Dioxide) - most common, forms residuals, can create DBP's 2. Iodine, I - high cost, dangerous to pregnant women 3. Bromine, Br - not commonly used, highly toxic and corrosive 4. Bases - high pH, can leave a bitter taste, tend to sterilize 5. Ozone - costly,high energy, leaves no residual, and difficult to store, but extremely effective at sterilization |
|
|
TYPES OF PHYSICAL DISINFECTANTS |
1. Ultraviolet Radiation UV - sterilizes, creates no residual, but creates no DBP's 2. Heat - expensive, sterilizes, not commonly used |
|
|
CHLORINE HYPOCHLOROUS ACID (HOCl) + HYPOCHLORITE ION (OCl-) |
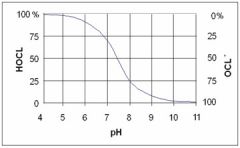
Depending on the pH of the water (see image below), the hypochlorous acid (HOCl) will partially dissociate to the hypochlorite ion (OCl-). Both hypochlorous acid and hypochlorite disinfect water but hypochlorous is acid is a more effective disinfectant |
|
|
CHLORINE PROPERTIES |
1. Greenish yellow in colour 2. 2.5 times heavier than oxygen 3. expands at 450 : 1 (liquid to gas) 4. non-flammable 5. supports combustion 6. will settle at the floor |
|
|
CHLORINE CHLORINE DIOXIDE |
Generated onsite by mixing Chlorine with with Sodium Chlorite (NaClO2) - creates no THM's - more effective disinfectant than Cl (pH 8-10), kills viruses and bacteria - less reactive than chlorine, so less is required to disinfect and provide residual |
|
|
ULTRAVIOLET RAYS (UV) |
UV is absorbed by cells of micro-organisms, damaging their genetic material - cells can no longer reproduce - UV treatment is costly and provides no residual - useful prior to chlorination as it avoids creation of DBP's |
|
|
ORP / REDOX PROBES |

ORP = "oxidation-reduction potential" Probes used to optimize chlorination,they measure the ability of chlorine to take on electrons (basically disinfect) - detect changes in millivoltage ** high ORP voltage = low levels of organics / high levels of free chlorine ** low ORP voltage = high levels of organics / high chlorine demand |
|
|
PRE-CHLORINATION |
Advantages: - control algal growths throughout system - limit mud-ball formation on filters - improve coagulation - reduce tastes & odors - increase chlorine contact time - can treat heavily contaminated waters Disadvantages: - creation of DBP's (THM's) - can create unwanted taste & odors if phenols are present in high numbers |
|
|
OZONE |
produced when O2 molecules are exposed to an energy source and converted into 03 (highly unstable) Ozone is the second most powerful oxidant in the world and can be used to destroy bacteria, viruses, and odors |
|
|
CHLORINE HYPOCHLORITE OCl- |
powerful disinfectant, used in treatment in two forms: 1. Calcium Hypochlorite Ca(ClO)2 2. Sodium Hypochlorite NaClO (bleach) **hypochlorite tends to raise pH **chlorine gas will lower pH |
|
|
PRODUCING CHLORAMINES 1. PRE-AMMONIATION |
1. pre-ammoniation followed by later chlorination PROs - produces mono-chloramines - fewer THM level than post-ammoniation - does not produce phenolic tastes CONs - not as effective at reducing tastes & odorscaused by diatoms |
|
|
PRODUCING CHLORAMINES 2. CONCURRENT ADDITION |
2. concurrent addition of ammonia & chlorine - added simotaneously into a rapid mix chamber ** produces the lowest level of THM's |
|
|
PRODUCING CHLORAMINES 3. PRE-CHLORINATION / POST-AMMONIATION |
3. Pre-Cl / post NH3 - chlorine added at head of plant (influent), produces free Cl residual in plant - ammonia added at effluent to produce chloramines throughout system PROs - high disinfectant within plant - effective at treating surface waters CONs - highest levels of THMs formed - chloramine residual less effective in system |

