![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
29 Cards in this Set
- Front
- Back
|
The kinetic theory of matter
|
• Particles of different substances have different sizes.
• Lighter particles move faster than heavier ones at a set temperature. • Particles move faster at higher temperatures • Solids: particles are very close and vibrate in fixed positions • Liquids: particles are further apart, have more energy and they can move around each other. • Gasses: particles are far apart, move rapidly and randomly |
|
|
Volatile
|
Liquids that evaporate at a low temperature.
|
|
|
Ion
|
Formed when an atom loses or gains an electron and becomes charged
|
|
|
Isotope
|
An atom of an element with a different number of neutrons than protons and hence a different mass number. Similar chemical properties because number of electrons are the same. Different physical properties because their masses differ.
|
|
|
Z
|
Atomic Number = number of protons
|
|
|
A
|
Mass Number = number of protons and neutrons
|
|
|
Compound
|
A pure substance composed of different elements chemically joined together in fixed proportion by mass.
|
|
|
Element
|
A pure substance containing only atoms with the same number of protons. Atoms have an equal amount of protons and electrons and thus a neutral charge
|
|
|
Mixture
|
Formed when two or more pure substances are mixed without chemically reacting.
|
|
|
Period Number
|
Number of shells that contain electrons = number of shell containing the valence electrons.
|
|
|
Valence Electrons
|
The electrons in the outermost shell containing electrons.
|
|
|
Electron Configuration
|
Describes the arrangement of electrons in energy levels (or shells) around the nucleus.
|
|
|
Electron shells
|
Electrons can be visualised as moving within a region of space surrounding the nucleus. The regions are called electron shells, labelled K, L, M, N, and numbered 1, 2, 3, 4. A definite energy level is associated with each shell, the one closest to the nucleus, K, being the lowest energy level. An electron has to gain energy to move further away from the nucleus. If it gains enough energy to completely leave the atom, the particle that is left is no longer neutral and is called a positive ion.
|
|
|
Formula for maximum number of electrons in a shell
|
2n² where n = number of shell. For first 20 elements the max number of valence electrons = 8.
|
|
|
Atomic Emission Spectrum
|
Produced by passing the light emitted by an element through a prism. Atomic emission spectra consist of separate lines of coloured light. Each line in an emission spectrum corresponds to one particular frequency of flight being given off by the atom; therefore each line corresponds to one exact amount of energy being emitted.
|
|
|
Periodic Table Groups
|
Columns of the Periodic Table. Each element in a group has the same number of valence electrons.
|
|
|
Alkali Metals
|
Group 1. React with water to form alkaline solutions. Very reactive. Low Density.
|
|
|
Alkaline Earth Metals
|
Group 2. First extracted from oxides in earth's crust. Less reactive than alkali metals.
|
|
|
Halogens
|
Group 17. So reactive that they don't occur freely in nature. Combine with different metals to form salts.
|
|
|
Noble/Inert Gasses
|
Group 18. Do not react readily. Exist as single Atoms. Colourless at room temperature with low melting and boiling points.
|
|
|
Metalloids
|
AKA Semi Metals. Have properties of both metals and non-metals.
|
|
|
Transition Metals
|
Large group of metals from group 3 to 12
|
|
|
Periodic Table Periods
|
Rows of periodic table. Elements in a period have their valence electrons in the same electron shell and share a gradual change in chemical and physical properties.
|
|
|
Lanthanides
|
Period 6. Rare earth metals.
|
|
|
Actinides
|
Period 7. Radioactive Elements.
|
|
|
Periodic table trend down a group
|
More electron shells mean bigger atomic size and because outer electrons are shielded by inner shells, they are less attracted to nucleus.
• metallic character increases & non-metallic character decreases • atomic size increases • reactivity Increases • electronegativity decreases |
|
|
Periodic table trend across a period
|
The attraction force of the nucleus increases with increasing number of protons. This pulls the electron shells closer to the nucleus
• metallic character decreases & non-metallic character increases • atomic size decreases • reactive at the start of the period, less in the middle and reactive at the end • fluorine = most electronegative. The further away an element is placed from fluorine in the periodic table, the less electronegative it is. |
|
|
Position of Hydrogen
|
• group 1 (alkali metals) - 1 valence electron that can be lost during reactions
• group 17 (halogens) - share many properties and can gain an electron during reactions • often placed away from any particular group |
|
|
Atomic Symbol
|
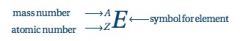
|

