![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
8 Cards in this Set
- Front
- Back
|
What do medical tracers use?
|
Beta or Gamma radiation
|
|
|
How does a tracer work?
|
A source that emits beta or gamma radiation is injected into the patient or swallowed.
Radiation moves around body and can be detected externally. radiographer can get a snapshot of its distribution. Computer converts to onscreen display shows radiation. can check organs. |
|
|
Does a tracer have a long or short life?
|
short!
|
|
|
Why would alpha radiation be bad for a tracer?
|
it would be stopped by tissue so never detected externally. also strong ionising power makes it really harmful for you insides.
|
|
|
What can Gamma radiation be used for?
|
Industrial tracer. same as body to look for cracks. Must have short half life.
|
|
|
How do scientists date rocks and specimens?
|
Measure the amount of radioactive isotope left in the sample. They know its half-life so can see how old it is.
|
|
|
What are igneous rocks and how can we tell how old they are?
|
They contain radioactive uranium which has a long half-life. It decays to become stable isotopes of lead. so can see how old by proportions of lead vs uranium.
Also same goes with potassium-40 to argon in Uranium. |
|
|
What is carbon dating and how work?
|
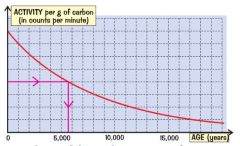
Carbon-14 is one ten millionth in living things at a constant level. when they die the trapped C-14 in wood, bones etc. is beta-emitting RADIOACTIVE ISOTOPE so decays over time.
The ratio of C-12 to C14 is fixed so comparing a living sample with dead can help estimate the age. |

