![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
100 Cards in this Set
- Front
- Back
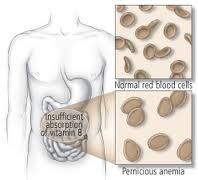
Pernicious Anemia
|
Lack of intrinsic factor leads to inadequate B12 absorption which causes DNA synthesis impairment resulting in megaloblastic anemia (ineffective hematopoesis)
|
|
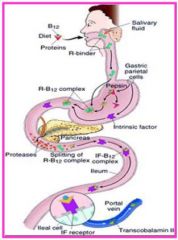
What cells normally produce intrinsic factor?
|
Gastric Parietal Cells
|
|
|
What are the three autoantibodies found in pernicious anemia?
|
1. Autoantibodies to gastric parietal cells (for screening, not specific)
2. Autoantibodies to IF (diagnostic) 3. Autoantibodies against IF-B12 complex receptor |
|
|
In what type of patient will all three pernicious anemia autoantibodies be found in?
|
Chronic Gastritis
|
|
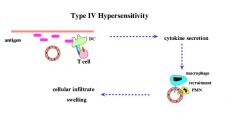
What cells primarily mediate pernicious anemia?
|
CD4+ T cells = Type IV Hypersensitivity
|
|
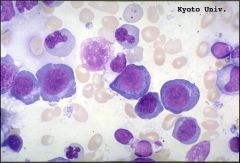
What do RBC look like in megaloblastic anemia
|
large erythrocytes; easily removed by phagocytes; ineffective hematopoiesis
|
|
|
Pathogenesis behind megaloblastic anemia
|
Vit B12/folate required for thymidine synthesis, DNA synthesis delated, delayed nuclear maturation compared to cytoplasmic maturation
|
|
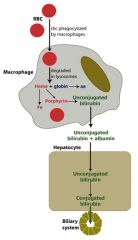
Extravascular hemolysis
|
Antibodies to RBC; opsonized for phagocytosis by macrophages
|
|
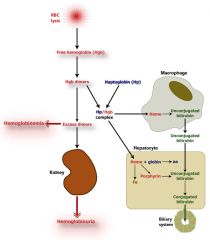
Intravascular hemolysis
|
Antibodies to RBC; complement binds by classical complement pathway; RBC lysed internally by MAC
|
|
Paroxysmal Noctural Hemoglobinuria
|
ACQUIRED deficiency; GPI linked proteins not synthesized; RBC sensitivity to alternative complement pathway activation
|
|
|
Ham's Test
|
Acidification (acid hyperactivates alternative complement pathway) causes visible lysis of RBCs from PNH patients; theses pts also lyse more at night bc sleep decreases blood pH
|
|
|
Function of DAF (CD55)
|
Protects RBC from hemolysis
Inhibits formation of C3 convertase; this inactivates (alternative) complement pathway In pts with normal DAF (GIP linked protein) RBC are not lysed |
|
|
Function of CD59
|
Protects RBC from hemolysis
Inhibits addition of C9 thus inhibiting MAC formation |
|
|
Immunohemolytic Anemia
|
Autoantibodies to RBC
|
|
|
Warm Antibody Type Immunohemolytic Anemia
|
IgG anti-RBC act as opsonins; RBC lose membrane, become spherocytes and are removed by the spleen (splenomegaly)
>30 yo |
|
|
Cold Aggulutination Type Immunohemolytic Anemia
|
IgM antibodies agglutinate RBC at low temp
> 60 yo |
|
|
Two mechanisms for drug induced hemolytic anemia
|
1. Drug acts as antigen; binds to RBC = hemolysis
2. Drug causes a break in tolerance (autoantibody against own Rh antigens, production ceases once drug is discontinued) |
|
|
Role of Coomb's Test
|
Diagnosis of immunohemolytic anemia; demonstrates presence of antibodies or complemnt on patient's RBCs
|
|
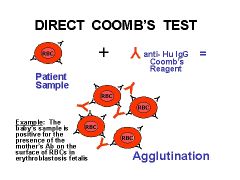
Direct Coomb's Test
|
RBCs from patient mixed w/ antihuman antibodies; agglutination = positive result
|
|
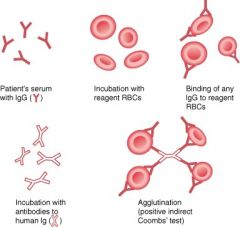
Indirect Coomb's Test
|
Serum from patient is mixed with normal RBCs and antihuman antibodies; agglutination = positive
|
|
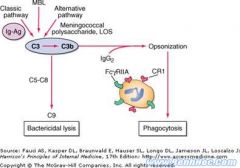
Describe the role of CR1 on erythrocytes
|
Ag-Ab complexes form in circulation w/ C3b; C3b binds to CR1 on erythrocytes; in spleen/liver phagocytic cells remove the immune complexes
|
|
|
Solubility of 02 relative to C02
|
C02 is 24x more soluble than 02
|
|
|
Dalton's Law
|
Percent concentration of each gas in a system must add up to 100%
|
|
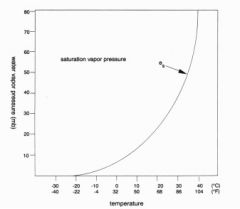
In alveolar air, oxygen is displaced from 21% (atmospheric conc) to 14%. Why?
|
Oxygen is displaced by water vapor (which increases from .5% to 6.2%)
|
|
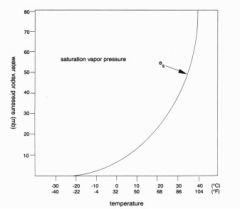
Relationship between temperature and water vapor pressure
|
Increased temperature = increased vapor pressure
|
|
|
Equation to calculate partial pressure in body fluids
|
(Atmospheric Pressure mmHg)(% Gas) = Partial Pressure mmHg
|
|
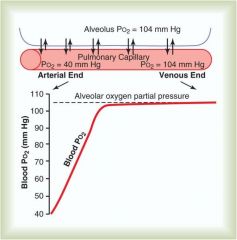
What does the rate of oxygen loading into blood in alveolar tissue tell us?
|
Since Blood P02 reaches almost 100% only 1/3 of the way down the pulmonary capillary, we could increase the blood flow (heart rate) significantly and still get 100% saturation
|
|
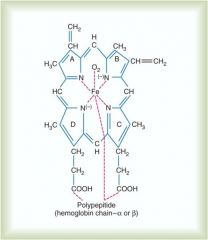
where does oxygen bind on the hemoglobin molecule?
|
Loosely binds to iron moiety; (remember carbon monoxide bonds 250x tighter than 02 to this site)
|
|
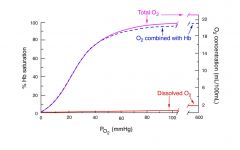
What is the blood's capacity to carry dissolved oxygen?
|
Very low
|
|
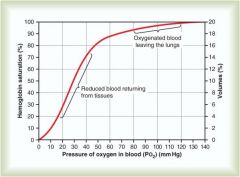
What is the P02 level in oxygenated blood leaving the lungs relative to reduced blood returning from tissues
|
Oxygenated P02: 80-120
Reduced P02: 20-40 |
|
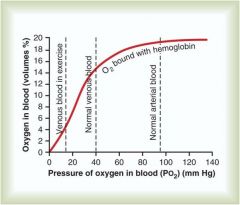
Compare the P02 in venous blood during exercise relative to normal
|
P02 in blood during exercise is much lower (0-18) relative to normal venous blood (20-40)
|
|
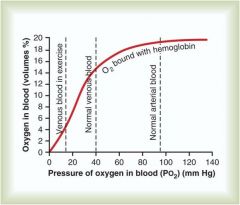
Relationship of P02 (mmHg) to percent 02 saturation of hemoglobin
|
Positive logarithmic relationship; increased P02 increases percent 02 saturation of hemoglobin
|
|
|
What is the P02 and Hb saturation of venous blood returning to lungs
|
40 mmHg and a saturation of approximately 75%
|
|
|
What is the P02 and Hb saturation of oxygenated blood leaving the lungs
|
P02 = 100%
Saturation = 100% |
|
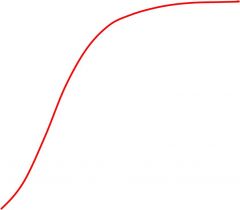
Relationship of PC02 to the hemoglobin dissociation curve
|
Increasing PC02 shifts the curve to the right, meaning for an increase in PC02 there will be a decreased 02 saturation of hemoglobin and the blood will carry less oxygen
|
|
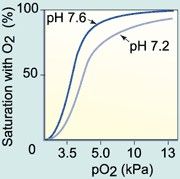
Bohr Effect
|
Lower pH causes a right shift in oxygen-hemoglobin saturation curve
|
|
|
Is pH lower in the tissue or lungs? Why?
|
Tissue; allows oxygen to be removed from blood as C02 is picked up by hemoglobin
In lungs pH increases which allows more 02 to bind to Hb |
|
|
Effects of temperature on Oxygen - Hb dissociation curve
|
Increasing temp shifts curve to right, decreasing temp shifts curve to left
|
|
|
Effects of 2,3-BPG on oxygen - hb dissociation curve
|
Increasing 2,3 BPG shifts curve to right, decreasing 2,3 BPG shifts curve to left
|
|
|
Difference between fetal hemoglobin and maternal hemoglobin 02 -Hb curve
|
Fetal hemoglobin is shifted to the right and is more saturated at lower P02s then maternal hemoglobin
|
|
|
Why is systemic arterial blood slightly below 100% P02
|
Freshly oxygenated blood is mixed with pulmonary shunt blood that has not been oxygenated
|
|
|
Relationship between blood flow and interstitial fluid P02 assumping normal 02 consumption
|
Increased blood flow increases interstitial fluid P02
|
|
|
Relationship between oxygen consumption and intestitial fluid P02
|
Increasing oxygen consumption decreases intestitial fluid P02
|
|
|
Relationship between C0 and Hb stauration
|
Very low PC0 will produce 100% Hb saturation
|
|
|
Relationship between blood flow and intestitial fluid PC02 in normal metabolism
|
Decreasing blood flow increases interstitial fluid PC02, increasing blood flow decreases interstitial fluid PC02 (but minimally)
|
|
|
How is C02 transported in the body
|
1. C02 in plasma = 7%
2. Hg-C02 = 23% 3. HC03- = 70% |
|
|
What ion replaces bicarboante to maintain electrostatic imbalance/electroneutrality of cell
|
Cl- (chloride shift)
Some water can also move into the cell, which indicates that in th venous blood rich in C02 the RBC may be slightly swollen due to increased movement of H20 into cell along w/ chloride - thus a slightly greater hematocrit taken from venous side relative to arterial side |
|
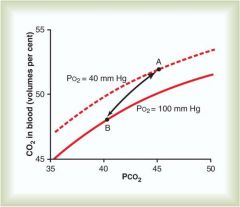
Haldane effect
|
Deoxygenation of the blood increases its ability to carry C02; conversely oxygenated blood has a decreased capacity for C02 (which allows C02 to be dumped into the alveoli for excretion)
|
|
|
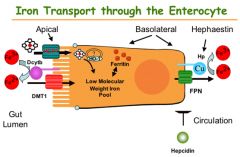
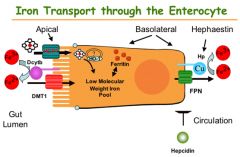
How is iron excreted
|
Menstruation, skin shedding, GI mucosa shedding
|
|
|
Transferrin (TF)
|
Binds to iron for transport
|
|
|
Where is msot iron found?
|
Bone marrow, mature erythrocytes (hemoglobin)
|
|
|
Where does iron absorption take place?
|
Duodenum (Heme = dietary, nonheme iron = cereals)
|
|

Ascorbic Acid (Vitamin C)
|
Reduces ferric acid to the more soluble and absorbable ferrous form
|
|
|
Ferric Reductase
|
Reduces ferric iron to the absorbable form ferrous
|
|
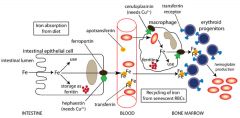
Ceruoplasmin & hephaestin
|
Ferrous converted to ferric state by ceruoplasmin in blood stream
|
|
|
Ferritin
|
Iron storage protein; binds ferric iron
|
|
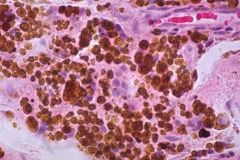
Hemosiderin
|
Denatured aggregate of ferritin; poor source of iron in low iron conditions
|
|
|
Elevated serum ferritin diseases
|
Hemochromatosis
|
|
|
Why does the body convert dietary ferrous iron to ferric iron and then back to ferrous iron for incorporation into heme?
|
If they body didn't convert iron to ferrric form for transport, it would produce superoxides and damage blood cells/vascular endothelium
|
|
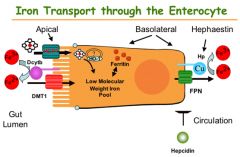
Ceruoplasmin
|
Converts iron from ferous to ferric state
|
|
Low ceruoplasmin levels
|
Wilson's disease; also associated with increased iron levels due to defective iron metabolism (ferrous -> ferric)
|
|
Aceruoplasminemia
|
Normal body copper metabolism but dramatic iron overload
Ceruoplasmins role is critical for iron metabolism and copper transport is secondary |
|
|
What determines how the cell responses to fluctuating intracellular iron concentrations?
|
Location of Iron Rsponse Elements and Iron mediated affinity of binding protein to these elements
|
|
|
Low soluble intracellular iron concentration; so cell wants iron uptake from plasma and restrict irion storage in ferritin; what does it do molecularly/
|
Translation of transferrin receptor mRNA is stimulated
Translation of ferritin mRNA is inhibited to reduce storage |
|
|
Heme
|
Fe + Protoporphyrin IX
|
|
How does the final product of heme synthesis regulate itself?
|
Heme inhibits the first commited step of heme synthesis (ALA synthase)
|
|
ALA Synthase deficiency
|
X linked Sideroblastic anemia
|
|
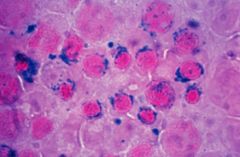
Lead Poisoning
|
Lead inhibits heme biosynthesis at the ferrochelatase step and porphobilinogen synthase step; hence synthesis of mature heme are deficient (symptoms = siderblastic anemia and porphyria)
|
|
|
Precipitation Reaction
|
Equimolar ratios of SOLUBLE antigen and antibody are mixed together, they form an insoluble latticework that precipitates naturally
|
|
|
Prozone
|
Antibody or antigen is in vast excess during a precipitation reaction and there is no precipitate
|
|
|
Zone of Equivalence
|
Max precipitate formed when ab=ag conc.
|
|
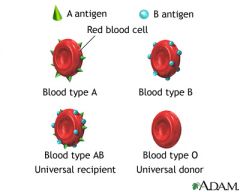
Where do antibodies of the ABO system come from?
|
These serum antibodies (usually IgM) occur naturally in respones to similar carbohydrate structures on normal GI flora
|
|
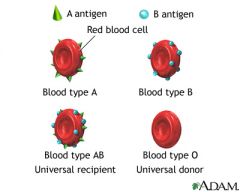
What kind of serum antibody will you find a patient with 00 blood?
|
Anti-A, Anti-B
|
|
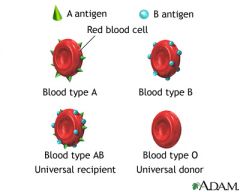
What kind of serum antibody will you find a patient with AA, AO blood?
|
Anti-B
|
|
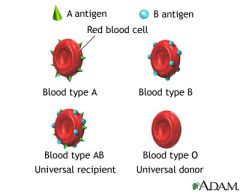
What kind of serum antibody will you find a patient with BB, BO blood?
|
Anti-A
|
|
What kind of serum antibody will you find a patient with AB blood?
|
|
|
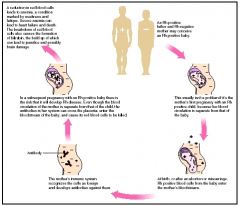
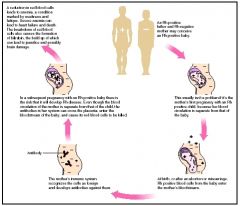
When are anti-Rh antibodies produced?
|
only when an Rh- individual is exposed to Rh+ RBC
IgG |
|
|
What type of hypersensitivity reaction is a transfusion reaction?
|
Type II Hypersensitivity
Pentameric IgM ab agglutinates RBCs; IgM also is good activator of the classical pathway of complement |
|
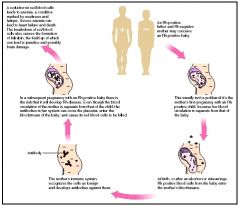
When is Rh+ compatibility a major concern?
|
Pregnancy; A pregnant RhD- woman w/ RhD+ baby can have a reaction during delivery and form anti-RhD IgG antibodies, which can cross the placenta and cause hemolytic diseaes of the newborn in subsequant RhD+ babies
|
|
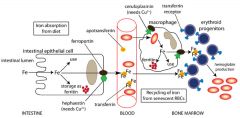
What would a lack of transferrin result in?
|
Atransferrinemia; severe anemia w/ iron overload in non-hematopoietic tissues
|
|
|
What two molecules make up Heme?
|
Fe-protoporphyrin IX
|
|
|
Reticulocytes
|
Immature RBCs
|
|
|
How is protein biosynthesis in reticulocytes regulated?
|
Heme levels activate heme kinase
|
|
|
How will high levels of heme affect protein biosynthesis in reticulocytes
|
Increase protein biosynthesis (alpha, beta globins)
|
|
|
How will low levels of heme affect protein biosynthesis in reticulocytes?
|
Decreased protein biosyntheis (alpha, beta globins)
|
|
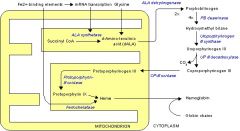
What is porphyria?
|
Disease caused by metabolites of the heme synthesis pathway, which accumulate and cause toxic effects w/ incomplete heme synthesis
|
|
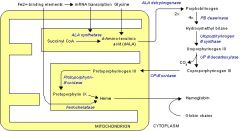
Porphyria Pathway
|
Glycine + Succ-CoA -> delta-ALA -> porphobilinogen -> (lots of porphyrins) -> porpphyrins -> protophyrin IX -> heme
|
|
What does the chrome in cytochrome indicate?
|
Heme; it's a part of CYP450; so things that induce CYP450 deplete heme stores, so the heme synthesis ramps up
|
|
|
Acute Intermittent Porphyria
|
AD enzyme activity which is usually enough to get by; but in things which induce CYP450 or other cytochromes (which depletes heme), most common porphyria
|
|
|
Myoglobin
|
Intracellular 02 storage protein
|
|
|
When does myoglobin release 02?
|
Low P02, indicating 02 deprivation
|
|
|
How does oxygen binding change the stereochemical configuration o fheme?
|
Oxygen binding pulls electrons from the iron and reduces its ionic radius, allowing it to move into the plane of the porphyrin ring. This pulls the proximal histidine (and the helix it belongs to), triggering a conformational rearrangement in the protein
|
|
|
What is the source of 2,3-BPG in RBCs?
|
2,3-BPG shunt
|
|
|
What is the function of 2,3-BPG
|
Allosteric effector of deoxygenated hemoglobin (in tissue); promotes the release of remaining oxygen molecules to tissues that need it most (increases in individuals living at high altitudes or chronic hypoxia) - increases the net delivery of oxygen per hemoglobin molecule
|
|
|
Which hemoglobin configuration is the low oxygen affinity state?
|
T State; Deoxyhemoglobin
|
|
|
Which hemoglobin configuration is the high oxygen affinity state?
|
R State; Oxyhemoglobin
|
|
|
Oxygen Binding Regulators; they're all negative regulates of 02 binding to heme
|
2,3 BPG
C02 Hydrogen Ion Chloride Ion |
|
|
What is the molecular basis of the Bohr effect?
|
Protonation of Hb stabilizes the T-state (deoxyhemoglobin), promoting oxygen release in the tissue beds
|
|
|
What is the molecular basis for the left shift of the oxygen dissociation curve for fetal and embryonic hemoglobin?
|
Gamma subunits of HbF/E has a lower affinity for 2,3 BPG (and thus stronger oxygen binding)
|
|
|
A 35-year-old man is brought to the ER after severing his right femoral artery in a woodshop accident. The patient requires a blood transfusion. Due to a blood shortage, however, the physician has no other recourse but to use outdated blood for the transfusion. Immediately following the transfusion the patient begins to show signs of hypoxia, slips into a coma, and dies. What happened?
|
the half life of 2,3-BPG in RBCs is about 6 hours
|

