![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
55 Cards in this Set
- Front
- Back
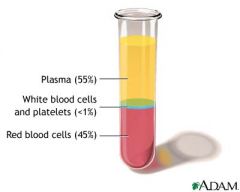
Composition of whole blood
|
Plasma 55%
Buffy Coat (white blood cells/platelets, trapped between plasma and RBC) <1% Red Blood Cells 45% |
|
|
Hematocrit of an individual w/ anemia
|
10-15%
|
|
|
Hematocrit of an individual with polycythemia
|
60-65%
|
|
|
Hematocrit
|
Proportion of blood volume occupied by red blood cells
|
|
|
Plasma
|
Contains water, proteins, lipids, electrolytes/nonelectrolytes, clotting factors which can be removed if the blood is allowed to clot and the fluid portion is removed (serum)
|
|
|
Serum
|
Plasma with clotting factors removed
|
|
|
Cation electrolye composition of plasma
|
Extracellular: Na+/Ca
Intracellular: K/Mg |
|
|
Anion electrolye composition of plasma
|
Extracellular: Cl, Hc03
Intracellular: P04, Protein |
|
|
Why is there is a small difference betwen the sodium concentration in the plasma vs interstitial fluid
|
Proteins that are held back primarily in the plasma have a negative charge and attract a small portion of the sodium molecules and retain them in the plasma slightly above what is seen in the interstitial space (Donnan effect)
Different charged substrates unable to pass through the membrane can create an uneven electrical charge |
|
|
What is the glucose concentration in plasma?
|
100 mg/dl
|
|
|
What is the normal urea to creatinine ratio in plasma
|
Urea: 15
Creatinine: 1.5 10:1 is the normal ratio, can be altered in some diseases |
|
|
Buffy Coat
|
Contains white blood cells and paltelets
Usually less than 1% of blood |
|
|
What are the dominant type of WBC in the buffy coat?
|
Neutrophils: 62%
Lymphocytes: 30% |
|
|
What is the lifespan of a RBC
|
120 days; this means we must have a mechanism in place to replace blood cells that die of old age or are lost due to a laceration or menstruation
|
|
|
Where are erythrocytes derived from?
|
Pluripotent hematopoietic stem cell
|
|
|
In an older individual, in what bones does erythrocyte production dominantly take place?
|
Vertebra, sternum, rib
|
|
|
In younger individuals, in what bones does erythrocyte productoin dominantly take place
|
Tibia/Femur (shaft of long bones)
|
|
|
Erythropoietin
|
Released in response to low tissue oxygenation; stimulates hematopoietic stem cell to increase production of RBC and increase oxygen delivery
|
|
|
What is the function of loose bonding of oxygen to iron in hemoglobin?
|
O binds loosely so it can be easily distributed to tissues
CO binds irreversibly to iron; is deadly even in relatively low concentrations |
|
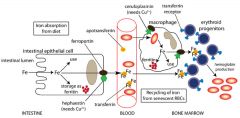
Transportation of iron
|
Iron is absorbed from small intestines where it is carried in the plasma by transferin which carries iroin to different tissues and is predominantly bound as ferritin in tissues; allows a constant source of free iron that can move back in forth between tissues and plasma
|
|
|
What is the relationship between blood viscotiy and hematocrit
|
Increased hematocrit exponentially increases the viscocity of whole blood
|
|
|
Blood Loss Anemia
|
After hemorrhage plasma is replaced more rapidly then erythrocytes
|
|
|
Aplastic Anemia
|
Lack of functioning bone marrow
|
|
|
Megaloblastic Anemia
|
Lack of vitamin B12, folic acid, or intrinsic factor result in low number of erythrocytes
|
|
|
Hemolytic anemia
|
Heredity anemias including sickle cell and erythroblastosis fetalis
|
|
|
Secondary Polycythemia
|
Exposure to high altitude results in increased levels of 2,3-BPG, which increases the production of RBCs
|
|
|
Polycythemia Vera
|
Genetic aberration in the hemocytoblastic cells that allows the continued production of RBC's even though an adequate number already exist
|
|
|
Rule of three
|
RBC = approximately FIVE x 10^6/mm^3
hemoglobin = approximately 15g/dl HCT = approximately 45% RBC value x 3 = hemoglobin hemoglobin x 3 = HCT |
|
|
Red Top Blood Collection Tubes
|
No anticoagulant; used to collect serum for chemistry; some red topped tubes have clot activators; clot time is approximately 30 min
|
|
|
Green Top Blood Collection Tubes
|
Contain heparin; heparin helps prevent cell breakdown and thus the release of K+ from RBC (remains as plasma and won't become serum since clot will not form)
Heparin used when you need an accurate potassium level |
|
|
Light Blue Top Blood Collection Tubes
|
Sodium citrate (1 part to 9 parts blood); tube must be completely filled to maintain correct dilution
|
|
|
What are the two major products of the pentose phosphate pathway?
|
NADPH and ribose-5-phosphate
|
|
|
What is the purpose of the HMP shunt/pentose phosphate pathway?
|
Provide a source of NADPH from an abundantly available glucose-6-phosphateNADPH is required for reductive reactions like glutathione reduction inside RBC);
|
|
|
In the pentose phosphate pathway what is the generated NADPH used for?
|
Fatty acid synthesis; glutathione reduction; other rxns
|
|
|
In the pentose phosphate pathway, what is the generated ribose 5-phosphate used for?
|
Nucleotide biosynthesis
|
|
|
What is the general rule differentiating NADPH and NADH?
|
NADPH: anabolic reactions (cholesterol biosynthesis, fatty acid biosynthesis)
NADH: catabolic reactions (glycolysis, TCA cycle) |
|
|
Gloucose 6 phosphate dehydrogenase deficiency
|
G6P generates NADPH which is necessary to reduce glutathione, which detoxifies free radicals and peroxides; decreased NADPH in RBCs leads to hemolytic anemia due to poor RBC defense against oxidizing agents (fava beans, sulfonamides, primaquine, antiTB drugs)
|
|
|
Epidemiology of glucose 6 phosphate dehydrogenase deficiency
|
Most common enzyme deficiency in world; 1/10 african americans carry a mutation in G6PD (associated with resistance to malaria)
Individuals often asyptomatic until provoked by illness or exposure to drugs |
|
|
Function of glutathione
|
Protects cells against oxidative damage
CYSTEINE residues between two glutathione molecules can form DIFULSIDE BONDS and be used as a site of oxidation and reduction |
|
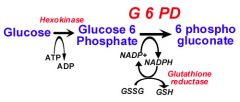
When might an individual with glucose-6-phosphate dehydrogenase deficiency run into problems?
|
Whenever there is an increase in reactive oxygen species; caused by medications, infections or FAVA BEANS
|
|
|
What is the result of severe oxidative damage in an erythrocyte?
|
Hemolysis
|
|
|
Heinz Bodies
|
Oxidized/denatured hemoglobin precipitated within RBC
|
|
|
Bite cells
|
Result from the phagocytic removal of Heinz bodies by macrophages
|
|
|
Phosphofructokinase enzyme deficiency (glycolysis) clinical presentation
|
Rhabdomyolysis and myoglobinuria, exercise intolerance, increased glycogen storage, hemolytic anemia, hyperuricemia
Classified as a glycogen sotrage disease type VII (similar to McArdles Type V) |
|
|
Pathogenesis of phosphofructokinase enzyme deficiency (glycolysis)
|
Catalyzes F6P -> F16BP which is a regulatory step for glycolysis
no glycolysis -> no ATP -> Na/K ATPase shutdown -> hemolysis ADP buildup -> increased AMP -> increased uric acid synthesis 3 isoforms of PFK: muscle, liver, platelet (M form is affected, RBC have both M and L form) |
|
|
Pyruvate Kinase Deficiency (glycolysis) clinical presentation
|
Second most common deficiency (after G6PDH)
Hemolytic anemia doesn't usually present till later in life; heterozygote asymptomatic PK mutations linked to increase malarial resistance |
|
|
Pyrovuate Kinase Pathogenesis (glycolysis)
|
PK catalyzes PEP -> Pyruvate
ATP made in this step Deficiency -> decreased ATP Some compensation by because of increase in 2,3-BPG (decreased affinity of Hb for 02) |
|

Methemoglobin Reductase (cytochrome B5 reductase) (methemoglobin reductase pathway) clinical presentation
|
Makes people blue; causes anemia
|
|
|
Methemoglobin Reductase (cytochrome B5 reductase) (methemoglobin reductase pathway) pathogenesis
|
MeR uses NADH from glycolysis to reduce ironb Fe3+(ferric heme - aka methemoglobin) to Fe2+ (ferrous heme) and ONLY ferrous heme binds oxygen; so knocking out MeR decreases the ability of oxgey binding = hemolytic anemia
Deficiency from MeR deficiency, genetically acquired mutant Hb allele or drugs Deficiency -> decreased oxygen carrying capacity |
|
|
2,3-Biphosphoglycerate Mutase (Rapoport-Luberin Shunt) clinical presentation
|
Deficiency rare; causes anemia
|
|
|
2,3-Biphosphoglycerate Mutase (Rapoport-Luberin Shunt) pathogenesis
|
BPGM catalyzes 1,3-BPG -> 2,3-BPG
2,3 - BPG is an allosteric effector of Hb (decreased Hb affinity for 02 - right shifts sat curve) |
|
|
Difference between ferric and ferrous state of hemoglobin?
|
Ferric: methemoglobin, can't bind oxygen
Ferrous: Can bind oxygen The reaction between the two is catalyzed by methemoglobin reductase (MeR), also called cytochrome b5 reductase |
|
|
What two things can you give to treat methemoglobinemia?
|
Methylene blue and ascorbic acid, which function as reducing agents to reduce ferric (Fe3+) to ferrous (Fe2+)
|
|
|
Methemoglobinemia
|
A deficiency in methemoglobin reductase (most severe form, present at birth)
|
|
|
What is the function of 2,3-BPG?
|
Decreases the affinity of hemoglobin for oxygen so it can be delivered to tissue sites with an increase oxygen requirement (high altitude, congestive heart failure)
Shifts Hb-oxygen saturatoin curve to the right |

