![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
12 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Acid
|
any compound that forms H ions in solution values below 7
|

|
|
|
Activation energy
|
energy needed to get a reaction started
|

|
|
|
Amino acid
|
compound with an amino group ( - NH2) on one end and a carboxyl group (- COOH) on the other end
|

|
|
|
Base
|
any compound that produces hydroxide ions (OH-ions) in solution; pH values above 7
|

|
|
|
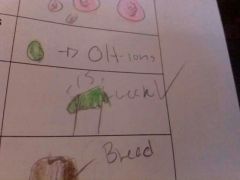
Buffers
|
weak acids or bases that can react with strong acids or bases to prevent sharp sudden changes in pH
|
|
|
|
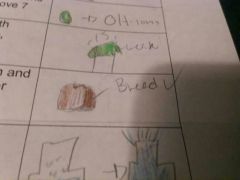
Carbohydrate
|
compounds made up of carbon hydrogen and oxygen atoms; major source of energy for the human body
|
|
|
|
Catalyst
|
substance that speeds up the rate of a chemical reaction
|

|
|
|
Chemical reaction
|
process that changes one set of chemicals into another set of chemicals
|

|
|
|
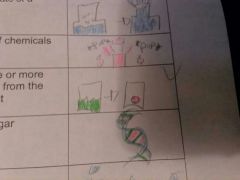
Dehydration Synthesis
|
a chemical reaction in which one or more molecules of water removed from the reactants to form a new product
|
|
|
|
Deoxyribonuleic acid (DNA)
|
nucleic acid that contains the sugar deoxyribose
|

|
|
|
Enzyme
|
protein that acts as a biological catalyst
|

|
|
|
Hydrolysis
|
is the chemical reaction or process in which a molecule is split into two parts by reacting with the molecule of water which has the chemical formula h2o. one of the parts gets an 08 H from the water molecule and the other part is an H from the water
|
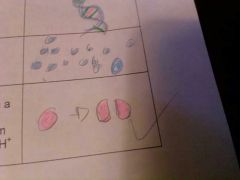
|

