![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
14 Cards in this Set
- Front
- Back
|
Element Box
|

|
|
|
Bohr Diagram
|
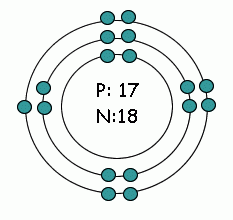
Protons and neutrons in the center,
Energy on energy levels (shells) in the electron cloud. Up to 2 electrons in the first energy level, up to 8 in the second, up to 8 in the third. |
|
|
Lewis Dot Structure
|

Shows only the valence electrons in the outer shell (energy level). Write the symbol for the element and draw dots to represent the valence electrons. Start on the right and work counter clockwise.
|
|
|
Periodic Table
|
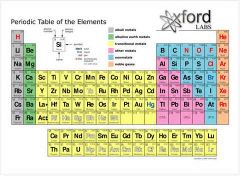
An organizational system for elements, places in specific places because of the way they look and act. Rows are left to right, and columns are up and down.
|
|
|
Alkali Metals
|
Very reactive metals that do not occur freely in nature. Malleable, ductile, good conductors of heat and electricity. Can explode if they are exposed to water.
|
|
|
Alkaline Earth Metals
|
Metals that are very reactive. Not found free in in nature.
|
|
|
Transition metals
|
Ductile and malleable, and conduct electricity and heat.
|
|
|
Lanthatnides
|
Transition metals found at the bottom of the periodic table. Shiny and reactive. Elements 58-71.
|
|
|
Actinides
|
Transition metals found at the bottom of the Periodic Table.
All are radioactive and therefore unstable. |
|
|
Other Metals
|
Ductile and malleable. Solid, have a high density.
|
|
|
Metalloids
|
Have properties of both metals and non-metals.. Some of them are semi-conductors (they can carry an electrical charge under certain conditions.
|
|
|
Non-metals
|
Not able to conduct electricity or heat very well. Very brittle and do not reflect light.
|
|
|
Halogens
|
Means "salt-former" and compounds containing them are called salts
|
|
|
Noble Gases
|
Do not form compounds easily. Happy/inert elements (full outer shells)
|

