![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
6 Cards in this Set
- Front
- Back
|
Vaporisation
|
When the sample is turned into gas(vaporised)
|
|
|
Ionisation
|
Gas particles bombarded with electrons to ionise them. Electrons are knocked of the particles, leaving positive ions.
|
|
|
Acceleration
|
Positive ions are accelerated by an electric field.
|
|
|
Deflection
|
Positive ions are altered with a magnetic field. Lighter ions have less momentum than heavier ions.
|
|
|
Detection
|
As the magnetic field strength slowly increases different ions (with a higher mass/charge ratio) reach the detector. As ions hit the detector they cause a current to flow.
|
|
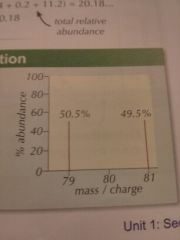
Find the Relative atomic mass of bromine
|
(50.5*79)+(49.5*81) / 100 = 79.99
|

