![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
22 Cards in this Set
- Front
- Back
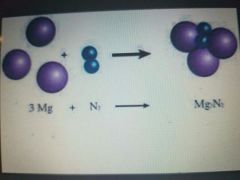
|
Synthesis reactions |
|
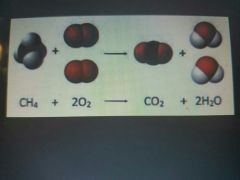
|
Combustion reaction |
|
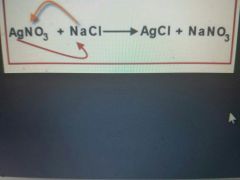
|
Double replacement reaction |
|
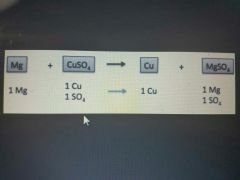
|
Single replacement reaction |
|

|
Decomposition Reaction |
|
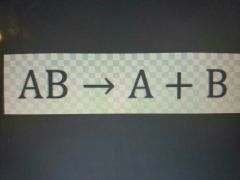
|
Decomposition Reaction |
|

|
Decomposition Reaction |
|
|
Synthesis reaction |
|
|
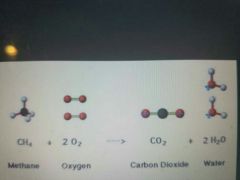
Combustion reaction |
|

|
Double replacement reaction |
|

|
Double replacement reaction |
|
|
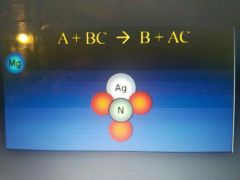
Single replacement reaction |
|
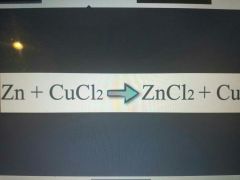
|
Single replacement reaction |
|
|
2K + 2Cl --> 2KCl |
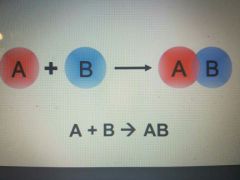
Synthesis reactions |
|
|
What is a Synthesis Reactions ? |
When 2 elements combine to form a product. |
|
|
What is a Decomposition Reaction? |
Opposite of synthesis The breaking of a compound into its components parts. |
|
|
NaCl --> Na + Cl |
Decomposition Reaction |
|
|
Combustion reaction |
Exothermic chemical reaction Initiated by heat acting w/ O2 & a fuel compound (hydrocarbon-gas or oil). Products are CO2 and H20. |
|
|
Single replacement reaction |
Involve ionic compounds. A more active metal reacting with an ionic compound contains a less active metal to produce a new compound. |
|
|
Cu(s) + 2AgNO3(aq) ---> Cu(NO3)2 (aq) + 2Ag (s) |
Single replacement reaction |
|
|
Double replacement reactions |
Involve 2 ionic compounds. + ion combine with - ion of another compound. Product: 2 ionic compounds that have switched partners. |
|
|
AgNO3 + KCl ---> AgCl + KNO3 |
Double replacement reaction |

