![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
103 Cards in this Set
- Front
- Back
|
Units of mesure for pressure
|
mmHg (760 mmHg = 1 ATM)
cm H2O (1033 cmH2O = 1 ATM) PSI (direct measurements pressure/area 14.6 PSI = 1 ATM) kPa (kilopascals 100 kPa = 1 ATM) ATM (weight of air column up to outer space) |
|
|
Characteristics of Blood: Viscosity
|
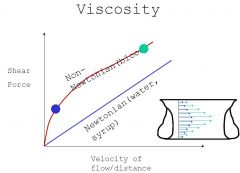
Viscosity: Measurement force requred to push fluid through a pipe or artery. Generally there is a linear relationship between force/velocity relationship, however blood is not a linear(newtonian) relationship like water. The reason is that blood is particulate, so that for instance when you are down at low velocity in the capillaries it acts more like mud than fluid (cell to pipe size match). As velocity rises in the large vessels it looks more like newtonian relationship. Note that the velocity at the edge of the artery is lower than that in the middle (although that difference is not as great in small vessels/low velocity). This principal explains why most of the myocardial flow happens in diastole (dialated vessels).
|
|
|
Characteristic of Blood: Shear
|
Blood Viscosity = 3.5 cenitpoise
Water Viscosity = 1.0 centipoise This is the drag on the endothelium that is produced by the flow of blood. The endothelial cells are actually transducers of shear force...they detect the force and the resultant change in shape triggering the relaease or supression of release of vasoactive compounds (NO, Prostaglandins, etc). Exceedingly high shear forces can strip the endothelium off of the vessel completely. In large arteries the avg velocity of flow in 20 cm/sec and endothelial shear is uniform/equilibrium. This is called flow mediated dilitation and is a measure of endothelial function. |
|
|
reactive hyperemia
|
Local incerase in blood flow due to the presence of lactic acid in muscle when flow (having been occluded) is restored.
|
|
|
Characteristic of Blood: Fluid Pressure
|
Manometer measures the height of a fluid column above a reference. Pressure = Height X Density...but as density is pretty constant we usually ignore density and relate pressure directly to height. The density of mercury is helpful because water is not dense enough to prectically take a measurement. This is the basis for the distance-Pressure relationship.
For a regular 6' tall person the heart is about 140 cm above the foot which would cause a pressure of about 2 PSI, but the valves that are placed at about 10cm increments breakup the column so that the actual pressure in a segment is less. Many things can break the valves down (like vericosities) and can lead to dilitation/edema. Static venous pressures can be important in many diseases including heart disease and preipheral vascular disease. Normal venous pressure should be 5-6, and can be dx as high by looking at the veins in the neck. |
|
|
Characterisitcs of blood: Fluid Dynamics
|
In large arteries velocity is about 20 cm/sec and endothelial shear is equilibrial. When velocity goes up, flow may go from laminar to turbulent. That turbulence makes noise which is the source of murmurs.
If a heart valve gets narrowed (stenosis) for instance, the velocity in the narrowed area increases. Flow = Velocity X Area, and V2 = V1 X A1/A2 (for a constant flow system, called the continuity equation). Among other things he indicated that if we know V1 and V2 we can calculate the difference in pressure such that Delta P = KV^2 as detected by doppler ultrasound (used to assess valve narrowing). Narrowins of Aortic valve- Age, coarctation Mitral valve- rhumatic fever Pulmonic valve- congenital Arteries- athlerosclerotic disease |
|
|
Doppler Ultrasound
|
dP = KV^2 where V = velocity in meters/sec, P = pressure in mmHg and K = 4.
velocity of 4 thought the aortic valve is then a 64 mmHg pressure drop and means that it is time to have surgery.... |
|
|
Vacular Resistance
|
Pouseuille Equation:
said that flow = pressure X radius (Pi R to the 4th) all divided by 8 X length X Mu (viscosity). Note that this is one reason that people with high viscosity due to protein accumulation are often hypertensive. The simplified form is Flow = K X dP X R^4 so that a small change in radius has dramatic effects and is finely regulated. Resistance = dP / Flow (R = 8 L mu / pi R^4). This is important in measuring pulmonary vascular resistance for example. We often also measure the systemic resistance which is normall 5X that of pulmonary...but may be much less in cases of sepsis for instance. |
|
|
Valve Area
|
Valve Area = Flow / K X Sqrt dP. K is a different constant for different valves...Kav = 44.5, Kmv = 38. Flow is measured in this case via doppler/echo. Again we can calculate the area as a means for surgical eval. Also, as we see pressure going up with the narrowing of the valve, we can see the backup of blood and can cause various edemas etc...intolerance to exercise/increases in flow, etc.
|
|
|
Cardiac pressures, Wiggers Diagram
|
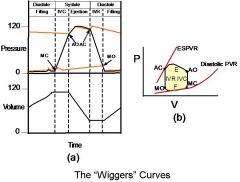
Understand the effect when the valves open and close, when blood is flowing where, and the relationship between the atrial and ventricular and pressure and aortic and ventricular pressure.
|
|
|
Venous Waves
|
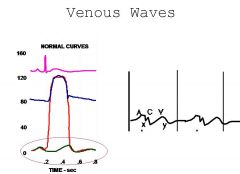
We can assess this by looking at the veins with the pt upright. We can actually see the waves going through corresponding to atrial contraction (a wave) before systole, and one other late in systole corresponding to the ventricular contraction ( V wave ). There is also a small C wave representing the kick/movement ofthe heart due to contraction. The descent of the A and V waves is called X and Y respectively. The pressures of the waves can be visualized on the wiggers diagram...
The waves change if we have regurgitation. For instance the V wave would dominate the venous pressure when we have Tricuspid vavle regurge (so all the ventricular pressure is going into the atrium and thus the veins). A-Fib produces only a V flutter, no A waves etc. We can also examine restrictive endocarditis this way, etc. |
|
|
Measuring CO
|
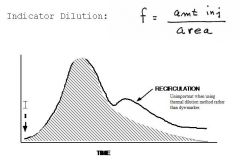
Flow = Vol/Time measured via:
Fick Method- If we send venous blood into the lungs with O2 we can use the O2 Sat before and after the lungs to measure consumption, and the 3 figures can be used to calculate flow. O2 consumption = Flow X AV differene...or CO = O2 compsumption / AV difference. There is a relationship between body area and O2 consumption so that we often do not calculate that directly. Ex. 300 (cons)/ 200 - 140 (in ml O2/L) = 5 L/min. Indicator dilution- Inject a marker into the blood stream and examine its spread to a remote location. The reason that we have 2 peaks is that we have recirculation, so often the hatched area has to be extrapolation. Flow = amt injected / Area under curve. We usually like to go at least one chamber down in the heart. Often we use Temp rather than color, by injecting cold saline into the right atrium and we see a slight dip in temp in the pulmonary artery. This is a thermal distribution, and note that the curve looks inverse and so we need to flip it around. Thus we now to measure CO use a Swan-Ganz catheter. It has a baloon to allow us to get it into the pulmonary artery and 2 injetion lument, one for the tip and one that will be at the atrial level. The pt can tolerate it for a few days at a time so it is useful. The measurements are taken electronically. Note that the catheter tip is also useful to sample mixed venous blood from the pulmonary artery for the Fick calculations. There is no recirculation this way as the temp evens out as the blood passes through the body so we get a single curve. The indwelling catheter can allow multiple measurements and tracking. |
|
|
Cardiac Conduction System
|
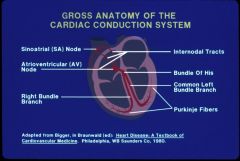
Crista terminalis (right atrium meets the VC), sits the SA nodes (from which we get sinus rhythm). It goes across the atrial to the AV node in the IV septum AV node sending it down the line. Note that at the end of the line it takes the ventricles about 0.12 - 0.2 seconds to contract)
|
|
|
Action Potential
|
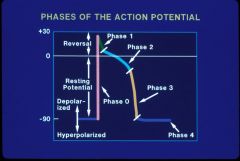
Voltage change over time, during depolarization and repolarization, generally in a single cell.
Controlled by opening and closing of ion channels in cell membranes Fast (Na+) or slow (Ca++) response of conduction velocity |
|
|
Excitability
|
Ability of a cell to respond to an external stimulus by depolarizing
|
|
|
Refractoriness
|
Inability of a cell to depolarize in response to an external stimulus (Phase 2-3 of the AP)
|
|
|
Conductivity
|
Property that a cell can transmit excitation to an adjacent cell (Phase 0 of the AP)
|
|
|
Automaticity
|
Property of certain cardiac cells that allows them to spontaneously depolarize (spontaneous excitation) (Phase 4)
|
|
|
Overdrive Supression
|
Property of normally automatic cells that, when excited faster than their own intrinsic rate, intrinsic automaticity stops temporarily (as long as the overdrive state persists) (Phase 4)
|
|
|
Action potential phases
|
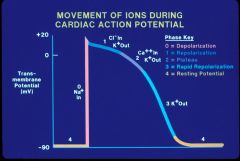
Phase 0
Action potential Upstroke All or None, initiated by reaching threshold potential. Upslope of phase 0 related to conduction velocity; fast vs slow response (Generally Na+ but sometimes Ca++ channel) Phase 1 Early Rapid Repolarization Ito Transient outward Current (K+) Inactivation of Ina, IK1, IKACT, IKACH, Inward rectifier currents Phase 2 Plateau Phase Slow inward Calcium Current ICaL Phase 3 Repolarization Slow and fast outward rectifiers, Iks, Ikr Phase 4 Resting membrane potential (-50 to –90 mV) K+ dependent, maintained by Na-K pump Spontaneous depolarization (If) causes automaticity (a current of NA leaking from outside in leading to threshold spontaneously is I sub f). |
|
|
Typical action potentials of cardiac tissues
|
There are diddrences in the shapes of the AP in different cardiac tissues, generally based on the cause of the 0 Phase.
The Atrial muscle, Ventricular muscle and Purkinje fiber all have rapid upstrokes as a result of Na channels. The Av and Sa nodes have slower upstrokes as phase 0 is mediated by Ca channels. The EKG is the summary of all the AP's measured from the surface of the body. |
|
|
Refractory Period
|
Divided into absolute (no AP no matter what) and Relative (AP possible but requires a stimulus that is greater that that of the resting state to get into AP.
|
|
|
EKG breakdown
|
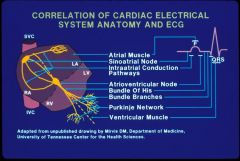
what we actually see is the p wave reflecting atrial cardiac muscle and a QRS reflecting ventriular cardiac muscle electrical activation. Many of the other structures are invisible from the surface (although we may be able to visualize them with catheters inthe heart, useful to Dx blocks etc).
|
|
|
Sinus node activity
|
Spontaneous Depolarization
Rapid rate of rise lowest resting potential dominant pacemeker as it si the fastest rate of spontaneous depolarization (the leakiest Ion channels) It is the major pacemaker current ofthe heart. Mediated basically by NA and modified by the autonomic nervous system (catecholamines etc...) SA-AV conduction is mediated by Na and perhaps some Ca channels as well. |
|
|
AV node actiity
|
Dependent on calcium channels (AV node) and sodium channels (His bundle and below)
Can be impaired partly or completely, intermittently or permanently Also can be affected by refractoriness (Potassium channels) (if the next stimulus finds that they cannot become excited, we may have problems. |
|
|
Causes of Arrhythmias
|

In general, abnormal rhythms are due to altered cardiac structure and/or function, due to a wide variety of conditions, E.G.:
Inherited (accessory pathway, channelopathy) Ischemia (CAD, ischemia or infarction) Valve disease Muscle disease Hypertensive heart disease Infections (HIV, Chagas disease, etc) Toxins (Alcohol, cocaine, etc), medications, drugs Metabolic (Electrolytes, etc), autonomic input Others These can cause altered conduction, refractoriness, automaticity, etc. and produce arrhythmias. |
|
|
Mechanisms of Bradyarrhythmias
|
Abnormal Impulse Formation or Abnormal Conduction
Essentially, failure of automaticity (If problem) to occur. Due to abnormal function of ion channels responsible for automaticity (If, others), and possibly channels involved in recovery (Ik, etc) Also involves failure of escape foci (a backup for the sinus node) to generate escape rhythms (subsidiary automatic cells that can “take over”, at lower rates, if sinus node fails) So if the depolarization is slowed, or if the cells are for some reason hyperpolarized or if the threshhold is somehow increased. At that point the ecctopic pacemaker takes over. Impulses need to conduct in several directions: to the atria to the AV node and from there elsewhere. Conduction can be impaired by malfunction of channels responsible for phase 0, or by markedly prolonged refractoriness |
|
|
Mechanisms of Tachyarrhythmias
|
Automaticity
Enhanced normal automaticity Abnormal automaticity Reentry Requires at least two pathways, with different refractory periods, at least one with areas of slow conduction/unilateral block Triggered activity Early afterdepolarizations, delayed afterdepolarizations Enhanced normal automaticity: Automatic depolarization (Phase 4), at rates faster than usual, usually by tissue that has normal intrinsic automaticity, due to increased rate related to activity of channels that are responsible for normal automatic activity. So a backup node hopped up on cocaine spikes too fast. Abnormal automaticity: Automaticity due to activity in channels or cells that normally do not produce automatic depolarization. Thus it overdrive supresses the sinus node. Symptoms- Syncope and presyncope. Palpitations |
|
|
Mechanism of Reentry Arrhythmia
|
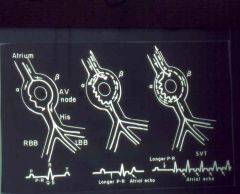
“Loop” or “short circuit” tachyarrhythmia
Usually caused by a premature beat that finds one limb refractory, and conducts down the other, more slowly conducting, pathway Slow conduction allows the refractory pathway to recover, therefore allowing retrograde conduction over that limb of the circuit. The impulse arrives at the “meeting point” of the two pathways, conducts down the slower pathway, and the reentry arrhythmia continues Thus for example a unidirectional block allows for the creation of an ectopic circuit that will go around and around and beat the heart with every turn. Causes: ischemia Valve Disease cardiac surgery electrolyte imbalance Possible clinical effects: Premature atrial depolarization supreventricular tachycardia ventricular tachycardia arrhythmia in preexcitation syndromes. In the diagram, B is fast coducting but has a long refractory time, A conducts sowly but recovers quickly. |
|
|
Mechanism of Triggered Activity Arrhythmias
|
Due to afterdepolarizations: “Upward” (positive) deflection of the action potential, during the end of phase 3 (EAD-early after depolarization) or during phase 4 (DAD -Delayed after depolarization).
Usually (but not always) due to abnormal function of various potassium channels If threshold is reached, a new action potential will develop, responsible for single extrasystoles, and also some sustained arrhythmias EAD can be the result of long QT syndrome which is a congenital defect in K channels DAD can be caused by digitalis toxicity. |
|
|
Sinus Node Dysfunction
|
Failure of automaticity (sinus arrest) or conduction (sino-atrial exit block)
Most common in elderly or patients with heart disease Offending drugs include beta blockers, calcium channel blockers, digitalis, most antyarrhythmic drugs, clonidine, lithium Results in pauses, slow heart rate Most common symptoms are fatigue, near syncope, syncope The key for weather we tx or no is weather or not they pauses are symptomatic |
|
|
Sinus Pause
|
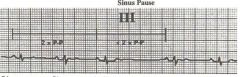
Sinus pauseis the result of intermittent failure of sinus node impule generation. This is manifest as a long RR cycle length (in thei tracing P to P interval) which is longer thant hte RR interval of the underlying sinus rhythm. There is no relationship between the cycle length of the pause and that of the intrinsic sinus rhythm. The p-p interval for the beats before and after the pause are less than 2 times the underlying sinus p-p interval.
|
|
|
Sinoatrial Exit Block
|
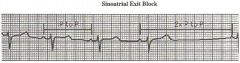
The new P wave comes right on time, so that this is not necessarilly due to sinus pause.
Failure of impule conduction from the sinous node intot he atrium resuls in sinoatrial block. This arhythmis is characterized by an absent P wave and a prolongation of the RR cycle length (measure P to P in this tracing), usually twice the underlying sinus P-P interval. This is a distinguishing feature form sinuspause in which the beat after the pause has a P-P interval less than the 2X sinus P-P. |
|
|
Tachy-Brady Syndrome
|
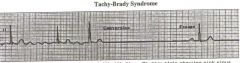
The atrium is fibrilitating and the myocytes are all working independantly in loops so the SA node is not funcitoning. If we can stop the looping and let SA wake up we can restore rhythm. Often indicated for a pacemaker.
|
|
|
First Degree AV block
|
All P waves conduct to ventricles, PR interval prolonged > 200 msec (takes too long). No QRS missing, may be a nice slim normal QRS indicating that the problem is in the Atrium. Wide QRS suggests that the disease is at the his bundle or below.
Can occur at AV node, His bundle, bundle branch, purkinje levels Clinical significance often minimal |
|
|
Second Degree AV block
|
Some P waves conduct, others do not
Wenckebach (Mobitz I): Progressive slowing of conduction (longer conduction time) prior to block, most often suggests AV nodal block. Long P wave. Mobitz 2: All conducted PR intervals are equal; most often block below His bundle High grade: 2:1, or occational conduction with no pattern. Can be AV nodal or below AV node In general, block at AV nodal level is not life threatening, is associated with normal (narrow) QRS; when block is second degree, exhibits Wenckebach pattern. May be physiologic (sleeping atheletes for instance), things that increase vagal tone like corotid massage, or drugs (dig, B blockers, Ca Antagonists, Antiarrhythmics). In general, block below the AV node can be life threatening, is associated with wide abnormal/wide QRS (bundle branch block); When second degree, exhibits Mobitz 2 pattern. Cannot be any escape rhythm. Higher risk of complete heart block More a eature of Na blockers and antiarrhythmic drugs. |
|
|
Compare 1st and 2nd AV Block
|
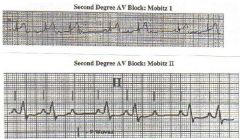
Note the differences.
|
|
|
Complete AV block
|
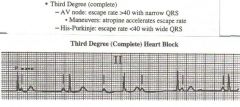
Definition: Complete absence of AV conduction when AV conduction would be expected to occur.
Usually, the atrial rate is faster than the ventricular rate. Can occur at AV node or below Can be present regardless of atrial rhythm (sinus rhythm, atrial fibrillation, Atrial Tachy, etc) Ventricular rate usually slow, regular (escape rhythm) causes: vagotonia valve disease cardiac surgery Metabolic disturbances Endocarditis Chagas diseasescleroderma sarcoidosis conective tissure disease lyme drugs trauma catheter ablation congenital Nice narrow regular QRS's with no relation to the P waves, no P regularlity, indicating that the problemis in the Atria. |
|
|
Single Extrasystoles
|
A single extra beat. Can be atrial (PAC) or ventricular (PVC). PAC's can conduct normally abnormally, or not at all. PVC's do not always look the same.
Most likely symptom is palpitations Often due to reentry, automaticity next most likely May be markers of underlying heart disease Usually do not require specific therapy and are not too clinically significant. |
|
|
Atrail Tachycardia
|
Paroxysismal Atrial Tachycardia PAT: Can be due to automaticity, triggered activity, microreentry, or macroreentry
HR most often 100-250 bpm. Can be with or without block Can be seen in “normal heart” patients, post-heart surgery, enlarged, “sick” atria, pulmonary disease, coronary disease, PE, hypertension, etc. Accelerated junctional rhythm where we have generally normal looking QRS's but no P waves indicating a problem in the his bundle or AV node. |
|
|
AVRT/AVNRT
|
Reentry tachycardias—reentry circuit is in AV node (AVNRT) or uses a bypass pathway for (usually) the retrograde limb back to the Atium(AVRT...can be congenital extra pathways).
Again requires 2 pathways, unidirectional block, a loop circuit, and something to initiate them. Often seen in patients without structural heart disease |
|
|
Preexcitation syndrome
|
accessory pathway conducitng in the anterograde and retrograde direciton makes the QRS funny and the PR interval short. Can be complex and dangerous
|
|
|
Atrial Fibrillatin and flutter
|
Very common
Any kind of heart disease; congenital predisposition, Aging Atria don’t contract effectively Ventricles may be fast (esp flutter) and irregular (esp fib) Symptoms can include palpitations, fatigue, etc. Again, ventricular responses with no nice P waves |
|
|
"Normal Heart" Ventricular Tachycardia
|
No structural heart disease
RV Outflow area most common site of origin Likely due to triggered activity Presents as PVC’s, nonsustained, or sustained VT Usually not life threatening Presents as palpitations, syncope |
|
|
Strutural Heart Disease Ventricular Tachycardia
|
Most commonly reentry (esp in post MI patients)
Most commonly arises in diseased areas of left ventricle Often fatal or life threatening Usually uniform (monomorphic) Presents as palps, syncope, cardiac arrest |
|
|
VT channelopathy in patients
|
Can include:
Long QT, Brugada syndrome, Catecholaminergic polymorphic ventricular tachycardia (CPVT), short QT Genetic disease of Ion Channels, usually no structural heart disease Gene mutations result in malfunction of ion channels, usually potassium channels, less commonly sodium or other ion channels The arrhythmia is due to triggered activity generally early after depolarizations. Polymorphic VT, Torsade de pointes rhythm...the points twist all over the place. Presents as syncope, cardiac arrest |
|
|
Syncope
|
Sudden unexpected loss of consciousness with spontaneous recovery.
Common in all age groups (~50% of pop will experience it) Causes that are heart related and pontentially life threatening: Aortic steonosis/decreased CO IHSS- Idiopathic hypertrophic subaortic senosis Primary pulmonary HTN/pulmonary embolism Left atrial moxoma Ventricular Tachy/fibrilation long QT syndrome/Torsade Infranodal AV block Preexcitation syndromes Usually Not dangerous: Sinus node dysfunction Av nodal block Carotid sinus hypersensitivity/syncope Atrial fib/flutter Tachy/brady syndrome Most SVT Most drug related bradycardia Most drug related hypotension vasodepressors situational Eval via Echocardiogram, Venous Doppler, VQ scan, Holter monitor, stress test, Echo...The point is to rule out the most dangerous stuff. |
|
|
Common Causes for Altered Automaticity
|

Common Causes for Altered Automaticity. Note that the increase/decrease is a change in rate of depolarization.
|
|
|
Therapeutic Choices for Arrhythmias
|
No Therapy
“Safe” medications (no major non-cardiac toxicity) Antiarrhythmic medications Catheter Ablation Antiarrhythmic devices Arrhythmia surgery |
|
|
Clinical Eval for Arrhythmia
|
History, Physical Exam
Symptoms, BP, HR, etc. ECG data (incl. Holter, telemetry, stress, etc) Response of arrhythmia to: Drugs, electrical stimuli Other lab data: EF, SAECG (special EKG processing), renal and hepatic function, etc. |
|
|
General Principals of Rhythm Management.
|
Make sure that the rhythm diagnosis is correct.
Tx underlying conditions that predispose to arrhythmias Educate and reassure the pt. Arrhythmias are often a manifestation of underlying heart disease. Therapy involves H/P and tx of the underlying problem is often more important than the arrhythmia itself. |
|
|
No Therapy
|
A good choice for arrhythmias that are not dangerous, not symptomatic (or minimally so), infrequent, and when the treatment is more trouble to the patient than the disease.
Examples: PAC’s, PVC’s, rare brief PSVT, asymptomatic short episodes of non-sustained ventricular tachycardia |
|
|
"Safe Medications"
|
These are meds that have little (or no) likelihood of serious non-cardiac toxicity. They work either by affecting the arrhythmia or by affecting the patient’s awareness of the arrhythmia.
Examples: Beta blockers for PVC’s; calcium channel blockers for PAT; Benzodiazepines for PAC’s or PVC’s |
|
|
Antiarrhythmic Drugs: Vaugn Williams Class I
|
Vaugn Williams Classes
Vaughn-Williams Class I: Drugs that primarily slow conduction; these primarily block sodium channels; subclassification IA, IB, IC according to degree of sodium channel blockade and amount of prolongation of refractoriness. IA: Quinidine, Procainamide, Disopyramide Moderate slowing of upstroke velocity, Some prolongation of refractoriness, Moderate time constant, use dependence (work more @ higher heart rates) IB: Lidocaine, Mexiletine Mild slowing of upstroke velocity, No prolongation of refractoriness, Very short time constant IC: Flecainide, Propafenone Marked slowing of upstroke velocity, Not much prolongation of refractoriness, Long time constant, use dependence |
|
|
Antiarrhythmic Drugs: Vaugn Williams Class II
|
Beta receptor antagonists. Major effect is to slow conduction or prevent arrhythmias by blocking beta adrenergic input. Subclassified according to beta 1 selectivity, presence of intrinsic sympathomimetic activity (ISA), other properties
Nonselective: Propanolol, Nadolol Beta 1 selective: Metoprolol, Atenolol ISA: Pindolol, Acebutolol Nonselective, also alpha blocker: Carvedilol |
|
|
Antiarrhythmic Drugs: Vaugn Williams Class III
|
Drugs that prolong refractoriness; these primarily block potassium channels; they exhibit reverse use dependence; a common toxicity is Torsade des Pointes/polymorphic VT
Examples: sotalol, dofetilide, amiodarone Amiodarone exhibits effects of all four classes |
|
|
Antiarrhythmic Drugs: Vaugn Williams Class IV
|
Calcium channel blockers
Dihydropyridine calcium blockers do not have significant rhythm/conduction effects Slow conduction by blocking calcium channels Effective for rhythms that depend on AV nodal conduction, or certain triggered activity related rhythms Examples: Verapamil, Diltiazem |
|
|
The Sicilian Gambit
|
A gambit is a strategy; Sicily is where a meeting of experts took place to discuss this method of drug classification:
Evaluate the mechanism of the arrhythmia Define the vulnerable parameter Identify target most likely to affect the vulnerable parameter Choose a drug that is likely to affect the target Example: AV nodal reentry Mechanism: Reentry Vulnerable parameter: Conduction Target: AV nodal or slow pathway conduction Drug: Metoprolol (or, ablation) |
|
|
Rhythm Control Devices
|
Pacemakers
Single chamber, dual chamber, CRT-P ICD’s (Internal Cardioversion defibrilator) Single chamber, dual chamber, CRT-D |
|
|
Pacemakers (add rhythm Strip)
|
Consist of a pulse generator with a power source/battery, leads or wires including a cathode and anode, and the body tissue completes the circuit. It can both send out pulses and can recieve/interpret input.
Uses: Prevent bradycardia Maintain AV synchrony (if there are inputs into the atria and ventricle) Provide rate response Faster pacing with certain physiologic inputs Able to interrogate, check, and reprogram noninvasively Stored data for arrhythmic events Last approximately five to ten years Paced Rhythm Recognition- We can see the paced pulse in the marker channel on the rhythm strip. When the heart is below a certain rate it paces but when the heart speeds up it senses the intrinsic P wave and just watches. Problems: Undersensing- The QRS is not sensed and so the pacemaker delivers a pulse when it shouldn't. Oversensing- An electrical signal other than the intended P or R wave is sensed and the pacemaker may be inhibited. Both can be adjusted by a programming change. Interference is also a concern. |
|
|
Pacemaker Interference
|
Safe Energy Sources:
Microwaves Household Appliances Remote Control Devices Small Electrical Tools Automatic Switching Devices Dental Equiptment X-ray/CT Scan. Electromagnetic Interference: EM fields that do interfere are radio-frequence waves. 50-60 Hz is the most troublesome. Few sources are at home, but several are in the hospital including: Electrocautery Transthoracic defibrillation Extracorporeal shock-wave lithotripsy Therapeutic radiation RF ablation TENS units MRI More rarely sources are in the environment including: Radar High energy systems in engines (alternator while engine is on) Other Transmissions |
|
|
Indications for Pacemaker Placement
|
Symptomatic bradycardias
Sinus node dysfunction, AV block Life threatening bradycardias (even if asymptomatic) Mobitz type II AV block, most acquired complete AV block, bradycardias that result in serious ventricular tachyarrhythmias |
|
|
Implanted Defibrillators (ICD)
|
Do everything a pacemaker does
Able to recognize and pace-terminate or shock terminate potentially fatal ventricular arrhythmias Data storage for arrhythmic events Multiprogrammable Last about five years Components: battery Sensing amplifier Control circuitry Defibrillation energy storage capacitors (800V shock from a 3V battery) High output switching circuit Pacing Circuitry |
|
|
Indications for ICD's
|
Secondary prevention
For patients who have experienced a sustained ventricular tachyarrhythmia, such as ventricular tachycardia, ventricular fibrillation, or cardiac arrest Primary prevention For patients who have not yet had, but are at high risk to develop, life threatening ventricular tachyarrhythmias; patients with serious left ventricular systolic dysfunction after a heart attack, or with LV dysfunction and clinical congestive heart failure |
|
|
Problems with ICD's
|
Infection
Lead migration or perforation Pneumothorax Bleeding Spurious shocks from ICD’s Many others Most are uncommon |
|
|
Resynchronization
|
For patients with CHF, LV systolic dysfunction, and bundle branch block on ECG (especially left bundle branch block)
Atrial synchronous ventricular pacing of both ventricles: RV from the apex, as with “usual” pacing, and LV using a pacing lead placed into a coronary sinus vein branch, or less commonly an epicardial lead Improves functional capacity Reduces CHF hospital admissions Improves quality of life Probably improves survival More complex, more difficult to implant, shorter battery life, and perhaps more problems than conventional pacemakers or ICD’s |
|
|
Catheter Ablation
|
Permanently cure arrhythmias
Renders drug therapy of the arrhythmia unnecessary Safe and effective, but not without possible complications Place electrode catheters into the heart Measure “normal” conduction properties Provoke the arrhythmia Study and “map” the arrhythmia to determine the mechanism and proper spot to target for the ablation Attach the ablation catheter to the radiofrequency generator and place it into the heart Determine the proper catheter placement Deliver energy, which causes a small burn in the heart; if located properly, the burn will interrupt a reentry circuit or scar an automatic focus and the arrhythmia will be eliminated |
|
|
Sinus Node Dysfunction (bradycardia) Tx
|
Eliminate offending drugs, conditions
No need for therapy if asymptomatic Pacemaker if symptomatic There are no real adequate drugs for this. |
|
|
AV nodal block (bradycardia) Tx
|
Elimiate offending drugs/conditions
No therapy if asymptomatic 1st or 2nd degree Pacemaker if symptomatic or complete (3rd degree) AV block. |
|
|
AV block below the node (bradycardia) Tx
|
Different prognosis (more risk of sudden death)
1st degree does not necessarilly need Tx. Most 2nd and 3rd degree require pacing regardless of symptamatology May need to do His bindle study to identify the site of block (electrophysiology study). |
|
|
Complete AV Block (bradycardia) Tx
|
Most require pacing.
|
|
|
Variation/classification of A-Fib
|
A-FIB: No obvious P waves between the QRS's...no baseline activity. Can be with slow or regular ventricular responses.
A-Flutter: Fast but very regular in morpholigy and timing. Lone A-Fib occurs in the absence of cardiac or other predisposing conditions. Acute AF generally lasts less than 48 hours Paroxysmal AF has transient recurrent episodes that revert to sinus rhythm spontaneously or with Tx. Presistant AF does not convert without intervention/cardioversion Permanent AF persists despite cardioversion. |
|
|
Conditions and Factors that predispose to A-Fib
|
HTN
Ischemic Heart Disease Advanced Age Rheumatic heart disease (esp Mitral valve disease) Nontheumatic valve disease cardiomyopathies CHF Congenital Heart Disease Sick Sinus Syndrome/Degenerative conduction system disease WPW syndrome Pericarditis Pulm Embolism Thyrotoxicosis Chronic Lung disease Neoplastic Disease Diabetes Postoperative State. |
|
|
Rhythm Vs. Rate control (with arrhythmia persistance)
|
Limited study (pt's with asymptomatic A-Fib pt).
Long term anticoagulation is required regardless of the strategy (age 65-75 or older with A-fib is a big risk for stroke). The studies indicated that there was no real benefit for one or the other. |
|
|
Acute management of A-fib
|
If they are hemodynamically unstable, shock them back to normal rhythm via defibrilator or cardioversion.
If stable: Anticoagulate with heparin (later with warfarin) rate control with B-blocker, Ca Blocker, Digitalis Consider cardioversion if <24-48 hours duration (electriacl or pharmacologic; If longer, TEE (trans esophageal echo to check for clots) first; or if >3 weeks of therapeutic warfarin. Eliminate offending substances (alcohol, etc...) |
|
|
Long term management of A-Fib
|
Rate control: Beta blockers, ca vlockers, Dig, Av ablation/pacemaker. Target of Resting rate 60-70, avg 80...Max of 120
Rhythm Control: Class Ic drug, Class III drug, Occationally Class Ia drug. Total elimination of arrhytymia may not be possible, But we want to limit the AF burden as much as possible. |
|
|
CHADS2
|
A-Fib and Flutter scoring to determine stroke risk/need for warfarin.
Congestive heart failure = 1 point Hypertension = 1 point Age > 75 (65-75 arguable) = 1 point Diabetes = 1 point Prior Stroke or embolic event = 2 points If the pt has... 0 points= ASA 325 mg, or no therapy 1 point = ASA 325 mg, or warfarin (INR target between 2-3) 2 or more points = warfarin |
|
|
paroxysmal SVT Acute Tx
|
Vagal maneuvers: carotid massage, valsalva, dive reflex
Drugs: Adenosine (prob drug of choice), beta blocker IV, calcium blocker IV, rarely others (Dig is not functional here) Overdrive pacing if available Electrical cardioversion |
|
|
paroxusmal SVT: Chronic Tx.
|
Avoid offending substances
Catheter ablation (esp with preeccitation syndromes) Beta blocker Calcium channel blocker Occasionally, antiarrhythmic therapy (esp if heart is structurally normal, the best drugs are flecanide and propophanone) |
|
|
PAC’s, PVC’s, Nonsustained VT
|
Need to evaluate for underlying structural heart disease that might predispose to arrhythmias
Avoid offending substances If asymptomatic, no need to treat specifically. If symptomatic, consider beta blocker, occasionally antiarrhythmic drug, possibly ablation; choice may depend on nature of underlying conditions |
|
|
Sustained ventricular arrhythmias: acute management (Tx)
|
If the patient is hemodynamically unstable (Hypotensive, unconscious, etc...), immediate cardioversion or defibrillation is required. V-tac can be stable...but V-Fib is a killer. The former has to be taken VERY seriously though.
If stable, get a 12 lead ecg; do a focused evaluation; the best drugs to consider are IV lidocaine or IV amiodarone; amiodarone is likely the more efficacious for most patients |
|
|
Sustained ventricular arrhythmias: chronic Tx
|
Generally life threatening
Usually best treated with ICD, unless a clear proximate cause can be identified and eliminated (the drugs are not effective in preventing these rhythms and preventing death) May also require preventitive antiarrhythmic drugs, occasionally ablation VT in patients without structural heart disease can often be ablated |
|
|
Patients at high risk of VT/VF
|
Most people do not survive their first sustained VT/VF.
Thus, ICD implant prior to an event in high risk individuals can be done. High risk groups include: Prior MI and EF <30% CHF class >/=2 and EF < 35% Some pts with long QTc, HOCM, ARVD, sarcoidosis, Brugada syndrome, etc. |
|
|
Torsades, Polymorphic VT
|
Acute Therapy:
IV Mg Increase HR (Isoproteronol, dopamine, pacing) Eliminate offending substances (esp drugs..ie someone with long QT syndrome can go into this with drugs like methadone?) Chronic Management: Beta blockers ICD implant for those at high risk. Avoid offending substances Occasionally, pacing to support HR. |
|
|
Congestive Heart Failure
|
Definition: an inability of the heart to deliver an adequate cardiac output to meet the metabolic needs of the body (results in a syndrome). Leads to an accumulation of blood in the vessels and fluid in the body tissues
Epidemiology: Approximately 4.5 million Americans have congestive heart failure, with 400,000 newly diagnosed patients each year and 200,000 deaths each year Once a patient has had an episode of symptomatic heart failure, 1 year mortality is 20%, 5 year mortality is approximately 50%. Incidence increases with age and is increasing as we become better at preventing death from coronary artery/valvular disease. It is responsible for 5% of all US hospital admissions. Symptoms: Decreased cardiac output: initially loss of reserve CO leads to dyspnea on exertion. Later reduced resting Co leads to organ under-perfusion and resting dyspnea, fluid retension, renal insufficiency... NA/Water retention leading to "congestion." Right sided congestion (pre Vena Cava), leg edema, hepatic congestion, increased intravascular volume, weicht gain. Left sided congestion (backs up from the left side of heart): Pulmonary Edema , SOB, orthopnea. Progressive chronic condition, with episodes of acute exacerbation. Causes: Coronary Artery Disease (ischemic cardio myopathy, or MI leaving scars) Dialated cardiac myopathy- low ejection fraction leads to cardiac dilitation (the heart becomes more spherical) valcular dysfunction Restrictive cardiomyopathy- the ejevtion fraction may be normal but the ability fot he heart to realx (via fibrosis of what have you) is limited. Constrictive percarditis.- The defect is not in the heart muscle but tye pericardium becomes thick and less compliant. |
|
|
Cardiomyopathy
|
Definition: Simply stated, “heart” + “muscle” + “disease.” The heart muscle, weakened by various causes, is not pumping enough blood (oxygen, nutrients) for the rest of the body to function normally. Note the common causes via %ages below...
Classification: General Cardiomyopathy categories Dilated CMP (32%)- leads to decreased Ejection Fraction, dilitation of the heart, decreased contractility Hypertrophic CMP- normal Ejection fraction but the heart hypertrophies inapprotpriately leading to thick walls. Restrictive CMP- Non distendable pericardium. Arrhythmogenic RV CMP- Right ventricular myocardium replaed by fat and scar Unclassified CMP Specific Cardiomyopathies (may fall into categories above): Ischemic CMP (40%) Valvular CMP (vol overload and regurg or pressure overload) (12%) Hypertensive CMP (11%) Inflammatory CMP (idiopathic myocarditis) Metabolic CMP General system disease Muscular dystrophy Neuromuscular disorders Sensitivity and toxic reaction Peripartum CMP |
|
|
Systolic/Diastolic Dysfunction, Right/Left Heart Failure
|
Definition:
Systolic Dysfunction: A failure of the pump to squeeze adequately Diastolic Dysfunction: Failure of the ventricle to relax adequately, preventing complete filling of the ventricle (ex restrictive CMP) “Left Sided” Heart Failure: Inability of the systemic ventricle to deliver blood to organs, reducing perfusion and causing pulmonary edema “Right Sided” Heart Failure: Inability of the pulmonic ventricle to deliver blood to the lungs, resulting in jugular venous distension, hepatic congestion, and lower extremity edema |
|
|
Classification for symptoms/severity of heart failure (NY heart association)
|
Graded by the degree of activity intolerance which correlates with levels of neuro-hormonal elevation and prognosis and NYJ class (catecholamines, inflamatory cytokines, aldosterone, norepi, angiotensin, etc...).
Class I: No symptoms, cardiac dysfunction with no limitation. Ordinalry physical activity does not cause symptoms. Prognosis is similar to normal folks. Class II: Symptoms After Moderate Exerttation, slight limitation in activity. Probably the majority of pt's with heart failure. Class III: Symptoms during activities of daily life (walking to the bathroom, cooking, light housework, dressing) leads to symptoms. Class IV: Symptoms at rest, or minimal activity (shortness of breath from brushing teeth, a conversation, seated in a chair). Prognosis, survival in a year might be about 50% |
|
|
Diagnosing CHF
|
History
Activity tolerance... Shortness of Breath Difficulty Sleeping (orthopnea/PND) Weight gain/Swelling in Dependent areas Dyspnea on exertion is the hallmark of heart failure. Left sided heart failure: cough (may be due to ace inhibitors), SOB/dyspnea/orthopnea. Right sided heart failure: Peripheral edema, abdominal bloating, anorexia, nausea. We may observe anxiety depression, decreased mental accutiry...due to brain underperfusion. Past Medical History Coronary Artery Disease Hypertension Diabetes Rheumatic Fever Social History Alcohol Tobacco Illicit Drugs Family History Medications Physical Exam VITAL SIGNS (BP, HR, RR, Temp, Pulse Ox) Jugular venous pulsation, carotid pulses Rales, crackles Extra heart sounds, murmurs Enlarged liver, abdominal bruits Lower extremity pulses, swelling X-ray Findings: Cephalizatin of vessels (they can start to see the vessels not just at the bottom of the lungs, but up towards the top of the heart Bronchial Cuffing Hylar vascular congestion Cardiomegaly (the heart should be less than half of the thorax width) Kerley B Lines- signs of edema between lobules of the lungs. Testing EKG, CXR Blood Tests (Blood counts, serum chemistries, thyroid studies, BNP [brain naturetic pepetid, made mostly in the left ventricle]) ECHO Specialty Testing: Stress Testing, Cardiac Catheterization, MRI |
|
|
Treatment of CHF (insert 24, consider re-listening to this section...??)
|
ID underlying causes and correct neurohomal derangement etc... This will prolong survival. Educate pt's (diet changes etc) and consider end stage therapies like transplant and mechanical assist devices.
Tx the reversable causes of cardiac dysfunction like revascularization in CAD, valve repair/replacement, heart rate/rhythm control, lifestyle changes etc... Diuretics: Reduction in circulating plasma volume by causing diuresis allows for more efficient functioning on the Starling Curve Digoxin A Na- K ATPase inhibitor, this medication has been a long mainstay of treatment for CHF A landmark trial published in the New England Journal of Medicine in 1996 (Digitalis Investigator Group, or DIG) demonstrated a reduction in hospitalizations, but no reductions in mortality with digoxin Renin- Angiotensin System Inhibition Angiotensin Converting Enzyme Inhibitors (ACEi) are the mainstay of treatment after a series of landmark trials in the 1980’s Angiotensin Receptor Blockers: Block the pathway further downstream; are probably as effective as ACEi, but are considered second line agents if patients don’t tolerate ACEi Aldosterone Blockers: Relatively new, potent therapies which provide further inhibition of the maladaptive effects of the RAS system Sympathetic Nervous System Inhibitors Beta blockers: long considered to be “contraindicated” in patients with congestive heart failure, a series of landmark clinical trials (MERIT-HF, Copernicus) have demonstrated that careful titration of these medications significantly prolong life Inotropes Dobutamine: a beta agonist used to increase cardiac output in decompensated heart failure, but drugs like this with the exception of Dig, increases mortality. Milrinone: A phosphodiesterase inhibitor that causes vasodilation and increase contractile activity used in decompensated heart failure Nesritide: A natriuretic peptide produced naturally in the ventricle in response to stretch, it causes sodium excretion and reduced heart work Devices Implantable Cardiac Defibrillator: Used to prevent sudden cardiac death from abnormal heart rhythms Intraaortic Balloon Pump: for cardiogenic shock Ventricular Assist Devices: devices to replace the pumping function of the heart Cardiac Transplant Note that the drugs that increase survival include ACE inhibitors, Beta blockers, Spironolactone and Angiotensin receptor blockers (really ace inhibitors). Also the combo of hydralazine and nitrates in african americans only. |
|
|
hemodynamic basis of heart failure
|
Preload- The right and left atrial pressures are referred to as filing pressures, or preload. Vol retention and reduced CO in heart fialure lead to increased preload.
Right increases due to valve regurge, right atrial insufficiency, etc lead to edema, weight gain, and liver congestion. Increase in left atrial pressure leads to pulmonary edema, cough, orthopnea, and paroxysmal nocturnal dyspnea (PND). Afterload- vasoconstriction in heart failure leads to increased afterload pulmonary vascular resistance (PVR) increases right ventricular afterload Systemic vascular resistance (SVR) increases left ventricular afterload (may not lead to increase in BP as flow may decrease. Cardiac Output: Healthy hearts can increas the CO on demand 4-5 times. The loss of that reserce leads to fatigue and dyspnea on exertion. In more severe cases the resting Co is insufficient, leading to symptoms at rest. Leads to dyspnea, renal insuffiency (intolerant of loss of CO) and fatigue. |
|
|
neurohormaonal Balance
|
In health
Vasoconstriction (Na/H2O Retention) Angiotensin II Norepinephrine Aldosterone Endothelin vasopressin Vasodilation (Na/H2O excretion) Bradykinin postaglandins nitric oxide natriuretic peptides In Heart Failure the vasoconstricter levels are raised to a high degree and overwhelm the dialators leading to vasoconstriciton and fluid/water retention. Regardless of the initial cardiac insult, there is a viscious cycle that ensues, This imbalance leads to weakening of myocytes, turns on apoptosis in cardiac myocytes, and thes increased imbalance etc. This leads to the hypertrophy and dilitation. |
|
|
Ventricular remodeling
|
heart attach leads to an area of initial infartcion. This area is replaced by scar and cytokines/inflamation increase the area of wound. The result is a local thinning of the wall, and the rest of the myocardium is effeted as a result of the neurohormonal imbalance.
Diastolic heart failure: Initially the sie and shpe of the heart would still look normal from the outside, but the walls are getting much thicker. The neruohormonal elevation will still lead to the same dialated spherical heart. |
|
|
Specific Physical Findings
|
Volume overload/Increased weight.
Right sided overload- elevated jugular venous pressure, hepato-jugular refles, abdominal distension, hepatomegaly, pitting edema. Left sided overlaod: rales, dullness to percussion (pleural effusions) Reduced CO- cool extremeties Cardiac Exam: S3 gallop (sign of high left atrial pressure, Loud P2 (pulm hypertension/pulmonic valve), murmurs, cardiomegaly (detect via lateral displacement of apical impulase on palpation). |
|
|
Mitral Stenosis
|
Etiology:
#1 Rheumatic Heart Disease (99% of MS) #2 Rheumatic Heart Disease #3 Rheumatic Heart Disease early on it causes valvulitis (rarely causes MS), late phase causes calcification (probably 2ndary to flow dynamics. Pure subvalvular stenosis is rare. Others (rare, unusual): Degenerative Calcification Hypereosinophilia Vegetation (very large) Myxoma (primary cardiac tumor, enlarges and restricts teh valve) Congenital Carcinoid Tumor SLE causes the valve to shrink/become stenotic. On X-ray we see prominant pulm vasculature, and we also see the large left atrium. |
|
|
MS Pathophysiology Hemodynamics (insert 8 and 9)
|
causes alterations in right and left ventricular fxn.
Murmur- the line of the atrial pressure gets very high, but people get used to it over time. When the pressure is high we heart the murmur, and at the end we hear the late acentuation (see wigers). We also can see this in pressure/vol loops where we have restriction of diastolic filling and decreased stroke volume. Classic Hemodynamics- Increased transmitral gradient during diastole. (may plateau with decreased cardiac output) Increased transmitral velocity. Reduction cardiac output. May only be apparent during exercise with moderate MS, With severe MS, @ rest, CO. ~ 3.0l/min mixed venous 50%., High Flow States are important. As this happens over such a slow period of time, people may have a fixed obstruction and not know it, esp if they do not exercise. Left atrial Hypertension Normal left atrial pressure: less than 12mmHg. Not usual for LA pressure of 25-28mmHg over time. Duration of diastolic filling is important. Results in pulmonary hypertension. Arrhythmias (Afib) Neurohumoral activation Thromboembolism Changes in Right & Left Ventricular function. Effects of left atrial hypertension: Dyspnea Pulmonary Edema Hemoptysis Pulmonary HTN Right heart failure Atrial fibrillation (along with HTN & CAD) Thromboembolic disease Pulmonary HTN: Dilatation of the pulmonary arteries. Reactive narrowing of the lumen. Hyperplasia of the intima in smaller arterial vessels. Right ventricular hypertrophy Chronic thickening of the wall of the capillary vessels in the lungs decrease the tendency for alveolar edema rupture and resultant hemoptysis. Left Ventricular Effects: Reduced left ventricular ejection fraction in 33%. Reduced preload. “Tethering” effect of the scarred valve. “Rheumatic factor” fibrotic scarring. Neurohumoral factors vasoconstriction and increased afterload. (often reversible). |
|
|
MS Pathophys A-Fib
|
Causes:
Increased right atrial pressures. Fibrosis of the atria, internodal tracts, and Sino-Atria node. Abnormalities in conduction velocities and refractory periods. Systemic hypertension promotes AFIB. “Self-promoting” (a viscious cycle of A-fib leading to dialation and dialation leading to a-fib) |
|
|
Ms pathophys Thromboembolism
|
20% of recently diagnosed MS have thrombus.
Increased thrombotic markers Risk Factors: 90% of pts. with thrombus have Afib. Degree of obstruction. Hx. of embolism Left atrial spontaneous echo contrast Present in 80% of pts. with MS & embolism Tx with coumadin |
|
|
MS Tx and Prognosis
|
baloon valvuloplasty
20% of recently diagnosed MS have thrombus. Increased thrombotic markers Risk Factors: 90% of pts. with thrombus have Afib. Degree of obstruction. Hx. of embolism Left atrial spontaneous echo contrast Present in 80% of pts. with MS & embolism Management: Reduce the effects of obstruction Beta-blockers to slow the heart Relieve congestion with Diuertics Reduce the effects of atrial fibrillation- Digoxin & Anti-arrhythmics ??? Prophylactic antibiotics Treatment of Complications Emboli Severe Pulmonary Htn Endocarditis Mitral Regurgitation Surgery: Commissurotomy or Mitral Valve Replacement Additional Surgery Plication of large left atrium Left atrial appendage ligation Maze procedure Other affected valves (especially TR) Surgery vs. Balloon Valvuloplasty |
|
|
Mitral Regurgitation (insert 30)
|
Etiology:
Leaflet Abnormalties Infectious Endocarditis Myxomatous Degeneration Mitral Valve Prolapse Collagen Vascular Disease Flail Leaflet Rheumatic Perforation Subvalvular Apparatus Ischemia Rupture Chordae Types: Acute Chronic compensated (has referenes to presentation of signs/symptoms) Chronic decompensated |
|
|
Acute Mitral Regurg (insert slide 27)
|
Increased LV Stroke Volume:
↓ Afterload ↑ Sacromere Stretch (Frank-Starling mechanism) Increased LV Diastolic Volume: Flow Back from MR Volume Forward Flow from pulmonary veins Leads to all kinds of problems as there is no time to compensate. |
|
|
Chronic Compensaged MR (insert 28)
|
Compensation via Eccentric Hypertrophy:
Individual myocytes lengthen by adding sacromeres. Chamber then dilates: resulting ↑ diastolic volume at lower pressures. Improved cardiac output. Increased chamber compliance. “Super”-normal LVEF: Relative normal cardiac output. Relatively asymptomatic patient. This is the best time to do surgery, offers the best cance for survival. |
|
|
Chronic Decompensated MR (insert 29)
|
Eventually the hypertrophy will not be enough, esp as regurg gets worse over time. Therefore they will eventually decompensate.
|

