![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
3 Cards in this Set
- Front
- Back
|
What makes Fe a transition element when Zn isn't? |
Transition elements have an incomplete d-orbital. Fe has 6 electrons in the d-orbital so is a transition element whereas Zn had a complete d-orbital with 10 electrons so isn't. |
|
|
Electron Configuration of Cr and Cu |
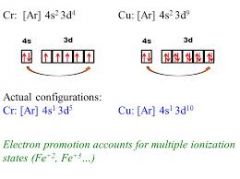
|
|
|
Precipitate Colours: Co 2+ + 2OH - --> Co(OH)2 Fe 2+ + 2OH - --> Fe(OH)2 Fe 3+ + 3OH - --> Fe(OH)3 |
Colbalt Hydroxide: Blue Precipitate Iron (II) Hydroxide: Green Precipitate (brown in air) Iron (III) Hydroxide: Rusty Brown Precipiate |

