![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
9 Cards in this Set
- Front
- Back
|
For transtion metals the 4s sub shell is always filled first, except for the elements of... |
Chromium and copper |
|
|
What is the definition for a transition metal |
A transition metal is one that can form one or more stable ions with a partially filled d-sub shell |
|
|
What is the electron configuration of scandium? |
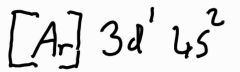
|
|
|
What is the electronic configuration of zinc? |
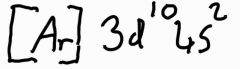
|
|
|
When ions form from transition metals, which subshell is removed first? |
When transtion metal atoms form positive ions, the s electrons are removed first, then d electrons |
|
|
What is ionisation energy |
Ionasation energy is the energy needed to remove an electron from an element |
|
|
All the transition metals have roughly the same first ionasation energies, what does this suggest |
Evidence that the transition metals all have similar electronic structure, and lose the first electron from the same shell (4s) |
|
|
Second ionasation energy increases across the elements. Cr and Cu are higher than you expect, why? |
This suggests that the second electron is taken from much nearer the nucleus (3d shell), so need more energy to remove it |
|
|
Explain 3rd ionasation energy for iron |
The third electron removed is the one in a paired 3d orbital. When there are two electrons in the same orbital they repel each other slightly, so it's easier to remove one of them |

