![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
41 Cards in this Set
- Front
- Back
|
What are the cardinal macroscopic signs of acute inflammation
|
Rubor (redness / erythema), calor (heat), dolor (pain), tumor (swelling / edema), loss of function
|
|
|
What are the major responses in acute inflammation
|
- Vasodilation (after an initial vasoconstriction to achieve hemostasis) with lymphatic proliferation
- Increased vascular permeability and loss of serum protein -> edema - Vascular stasis (congestion) -> increased WBC margination - Leukocyte extravsation |
|
|
What are the steps of WBC extravasation
|
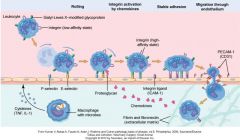
Blood stasis -> rolling of WBC on endothelial wall / margination -> weak endothelial adhesion via selectins -> integrin activation by chemokines -> WBC adhesion -> diapedesis through interendothelial junction -> chemokine gradiant to site of injury
|
|
|
What is the first migratory cell to arrive at and take a role in acute inflammation
|
Neutrophils. Local killing and degradation of bacterial macromolecules via phagocytosis and release of superoxide radicals and produce several pro-inflammatory cytokines. |
|
|
What are the major pro-inflammatory cytokines created by neutrophils
|
IL-1a, IL-b, IL-6, and TNF-a. |
|
|
What is the role of macrophages in acute inflammation
|
Early recognition of inflammatory stimuli; early source of pro-inflammatory cytokines; clean up of necrotic pmns; Release of anti-inflammatory mediators; debridement (phagocytosis, dissolution of ECM); stimulate fibroblasts later on
|
|
|
What are the major pro-inflammatory cytokines created by macrophages
|
Pro-inflammatory cytokines (IL- b, IL-6 and TNF-a), PGs and GFs (PDGF, TGF-a)
|
|
|
Is Interleukin-10 pro or anti-inflammatory
|
Anti-inflammatory
Depresses production pro-inflammatory cytokines and chemokines including TNF-A, IL-1, IL-6 & Il-8, by inhibiting translocation of nuclear factor kB (NF-kB) and promoting degradation of mRNA. |
|
|
How long does it take for neutrophils to be replaced by macrophages at a site of inflammation
|
24-48 hours.. Macrophages phagocytose apoptotic neutrophils in tissue.
|
|
|
What function do lymphocytes play in inflammation
|
Mostly acquired immunity but also early protective inflammatory responses (cell med immunity).
Helper (CD 4+) & cytotoxic (CD8 +) T cells major components cell-med imm. CD4+-> Th-1 &Th-2. |
|
|
What are the different functions of Th-1 and Th-2 cells
|
Under influence IFN-g & IL-2, T-cells -> Th-1 cells with characteristic cytokine profile (IFN-g and IL-2). IFN-g -> production of IG-G (IGg)2a by B-cells.
Th-2 function in helminthic infections and allergic reactions. Th-2 produce IL-2, IL-5, Il-10, IL-13. Overall this causes suppression of innate macrophage function, an increase in IgG1 and IgE production and eosinophil activation. |
|
|
What function do Mast cells have in inflammation
|
Ubiquitously distributed in all organs and degranulate in response to physical trauma, complement factors, microbial products or neuropeptides. Overall, degranulation enhances the local inflammatory response.
|
|
|
What is the difference between pathogen associated molecular pathogens (PAMPS) and danger associated molecular pathogens (DAMPS)
|
PAMPS: highly conserved microbial molecules, recognized as foreign by the host. Includes LPS, lipotechoic acid, peptidoglycan and microbial oligonucleoetodes.
DAMPS: endogenous molecules such as fibrinogen, which alert the body to cellular damage initiated by infectious or non-infectious agents. Both PAMPS and DAMPS signal the immune system by interacting with cell surface receptors See Figure 1-2, Page 4. |
|
|
What are toll-like receptors
|
Transmembrane proteins that initiate intracellular signaling cascades to activate nuclear factor-kB and result in altered gene transcription. Bind to ligands on PAMPs and DAMPs
|
|
|
What are tachykinins
|
Neuropeptides released from peripheral neurons after stimulation or trauma of sensory neurons. E.g. substance P
|
|
|
Name the two major vasoactive amines in inflammation.
|
Histamine -> mast cells Serotonin -> mast cells, basophils, neuroendocrine cells |
|
|
What are cytokines
|
Diverse group small soluble proteins that act as intercellular messengers during physiologic processes. Pro-inflammatory cytokines (e.g. TNF-a, IL-1b, IL-6 ) increase innate immune response Anti-inflammatory cytokines (e.g. IL-10, IL-1ra) attenuate the responses. |
|
|
What is the role of Tumor Necrosis Factor-a (TNF-a) in inflammation
|
1st pro-inflammatory mediator - Initiates production of pro-inflammatory cytokines (i.e. IL-6), reactive O2 intermediates, chemotaxins & endothelial adhesion molecules -> invasion of cells at site of inflammation Also activates NK cells, proliferation of cytotoxic T-cells and T-cell apoptosis |
|
|
What is the role of interleukin-1 (IL-1) in early inflammation
|
One of the 1st pro-inflammatory mediators -- Initiates cellular signaling pathways. Similar to TNF-a |
|
|
What is the role of IL-6 in inflammation
|
Initiation of hepatic synthesis of APPs and influences the proliferation of lymphocytes. Also has a compensatory down-regulation effect Increases in plasma proportionately with duration and severity of the condition. = can be used as prognostic and diagnostic indicator |
|
|
What are chemokines
|
Chemotactic cytokines responsible for attraction of cells across concentration gradient. Peak shortly after TNF-a and IL-1, along with IL-6. CXC and CC chemotaxins most involved in pro-inflammatory response to trauma of infection. CXCL8 (aka IL-8) attracts neutrophils and promotes production of other inflammatory mediators. |
|
|
What is the role of IL-10 in inflammation
|
Produced primarily by CD4+ and Th-2 cells, monocytes and B-cells. Depresses production of TNF-a, IL-1, IL-6 & Il-8, by inhibiting translocation of nuclear factor kB (NF-kB) and promoting degradation of mRNA. Down regulates Th-1 cytokines, promotes shedding of TNF receptors into systemic circulation and inhibits Ag presentation by macrophages and dendritic cells. |
|
|
What are Eicosanoids
|
Lipid mediators that are rapidly created from membrane phospholipids and exert effects locally. Their precursor is the fatty acid, arachadonic acid (AA). |
|
|
How do glucocorticoids suppress inflammation
|
*Decrease phospholipase A2 expression required for release of AA.
*Upregulate genes encoding anti-inflammatory proteins that inhibit AA release from PL. |
|
|
What function do PGs play in inflammation
|
*Chemotaxins for leukocytes *Induce vasodilation *Contribute to the pathogenesis of pain and fever. *Produced in COX pathways, where AA metabolism is catalyzed by COX-1 (constitutively expressed) and COX-2 (induced: trauma, growth factors, pro-inflammatory cytokines, other mediators). |
|
|
What is thought about selective COX inhibition
|
Inhibition of COX-1 causes gastric ulceration and should be spared inhibition of COX-2 alone may increase risk of cardiovascular and cerebellar vascular event -> no evidence yet of this in dogs so COX-2 selective agents are still a good option |
|
|
Leukotrienes are produced in the LOX pathway prostaglandins are produced in the COX pathway-true or false
|
True, COX inhibitors are used to reduce the production of the PGs. |
|
|
What function do leukotrienes play in inflammation
|
Pro-inflammatory mediators of leukocyte trafficking and blood flow. Secreted by leukocytes; peptidoleukotrienes provoke vasoconstriction, bronchoconstriction, and increased venule permeability. e.g. LTB4 potent chemotactic agent and activator of neutrophils, potentiating extravasation, degranulation and production of free radicals. |
|
|
What are pro resolution eicosanoids
|
They halt neutrophil infiltration, activate macrophage phagocytosis, increase clearance of phagocytes, and stimulate expression of molecules involved in antimicrobial defense lipoxins -> increased by aspirin; attenuate the effect of leukotrienes resolvins and protectins -> derived from Omega – 3 fatty acids and promote resolution of inflammation |
|
|
Where is platelet activating factor produced
|
It is produced by numerous cells and stimulated by multiple inflammatory mediators |
|
|
There are many effects of platelet activating factor. Name three
|
*Stimulation of arachidonic acid release and increase of eicosanoid production *direct pro-inflammatory effects on neutrophils: enhanced neutrophil adhesiveness, enhance neutrophil motility, primes neutrophils for degranulation. *Aggregate and degranulate platelets. *Degranulation and production of reactive oxygen species by eosinophils *increased vascular permeability. *Bronchoconstriction. *Pulmonary vasoconstriction |
|
|
What are the effects of reactive oxygen species
|
*Antibacterial defense (used in phagocytic oxidative burst), *intracellular signaling (induction of pro-inflammatory cytokine synthesis)
*pathologic tissue damage (reperfusion injury, pancreatitis, surgical trauma, abdominal adhesions) |
|
|
Is Nitric Oxide (NO) anti-inflammatory or pro-inflammatory
|
BOTH. Depends on concentration and mode of production. Produced by Nitric Oxide synthase.
constitutive = protective Basal levels protective anti-inflammatory effect on GIT, constitutive NO suppresses action of NF-kB, a transcription factor necessary for the expression of inducible NO synthase. Constitutive + out-of-control inducible = damaging High levels damaging effects on local tissues, perpetuate inflammatory cycle due to reactive nitrogen species (RNS) derived from NO |
|
|
What is the primary physiologic function of NO
|
Regulation of vascular tone & immune defense
Produces vasodilation by diffusing into smooth muscle cells and indirectly initiating intracellular signaling events, leading to smooth muscle relaxation. See Figure 1-4 Pg 10. |
|
|
What function does CO play in inflammation
|
Anti-inflammatory (impairs production, differentiation and activation of inflammatory cells, cytoprotective). |
|
|
What are negative acute phase proteins (APP)
|
Proteins active in regulating hemostasis (albumin); decrease by at least 25% during inflammatory response |
|
|
What are positive acute phase proteins
|
Role is to enhance protective host responses by minimizing tissue damage and enhancing repair processes. Increase in plasma concentration by at least 25% during an inflammatory response. Increase within hours and peak within 24-48 hours and, elevated as long as inflammatory stimulus maybe useful as diagnostic or prognostic markers e.g. C-reactive protein, Serum Amyloid A, Serum Amyloid P, Complement Proteins, coagulation factors |
|
|
What are the three routes of complement activation
|
*Classical – Immune complexes
*Lectin – Lectin proteins + pathogen surface carbohydrates *Alternative – Contact with foreign microbes *Result of each is a membrane attack complex -> cell lysis |
|
|
Whatare the parameters of systemic inflammatory response syndrome (SIRS) in humans?What does it indicate? Is this the same in dogs?
|
–Aberrations in body temperature, heart rate, blood pressure, respiration, whiteblood cell counts. – Diagnosis of sepsis when accompanied by nidus of infection. – Not really – too much variation in physiologic normalsragmenti`BC9 |
|
|
What is MODS
|
Progressive dysfunction ≥ 2 organ systems not involved in initial insult; probably secondary to major self-destructive information increasing incidence due to protracted patient survival |
|
|
What organ is believed to be the mediator driving MODS in the dog
|
GIT; reperfusion-mediated oxidative injury to gut epithelium = major source of mediators driving dysfunction of distant organs. |

