![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
23 Cards in this Set
- Front
- Back
|
Power radiated |
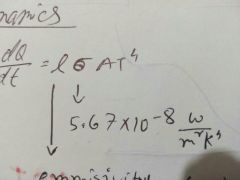
|
|
|
Value of sigma |

|
|
|
Wavelength of light most emitted from a black body |
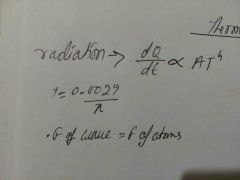
|
|
|
Black body radiation curve |
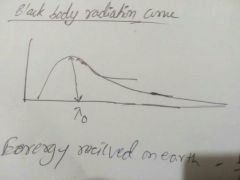
|
|
|
Energy received by earth from sun |
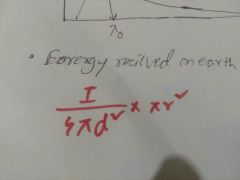
|
|
|
Net Power transferred by radiation |
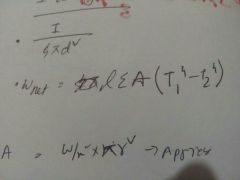
|
|
|
RMS in terms of vx vy vz |

|
|
|
Standard RMS formula |
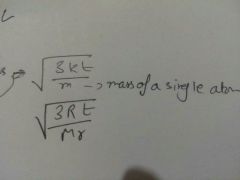
|
|
|
Value of k |
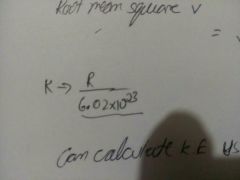
|
|
|
Kinetic energy of any gas of one atom and one mole |
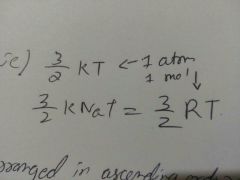
|
|
|
RMS of 9 molecules given their velocities |

|
|
|
Most probable velocity |
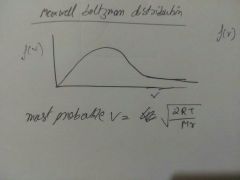
|
|
|
P in terms of RMS velocity |
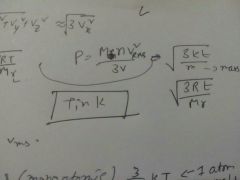
|
|
|
Average velocity |
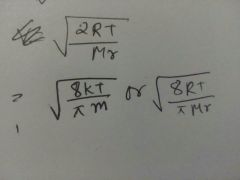
|
|
|
Radius of a hydrogen atom |
0.053nm |
|
|
Mean free path |

|
|
|
Time between collisions |

|
|
|
Delta p,v and t for free expansion |
All zero for ideal gas. Delta t positive for real gas. |
|
|
Delta s for reversible process |
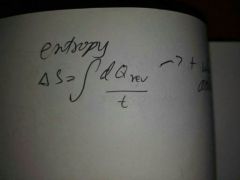
|
|
|
When delta s is positive and when it is negative |
Just like delta t. Positive if increased. |
|
|
Delta s for free expansion |
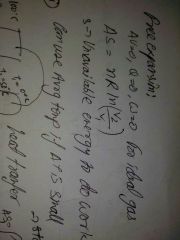
|
|
|
What to use as delta t to find s |
AAVerage t for small change |
|
|
WD in thermodynamic cycle and is sign |
Area under the graph + if clockwise |

