![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
15 Cards in this Set
- Front
- Back
|
The name of the energy needed to melt or freeze 1 g of water at the freezing point is called....
|
heat of fusion
|
|
|
The name of the energy needed to condense or boil 1 g water at the boiling point is called...
|
heat of vaporization
|
|
|
While freezing or melting is occurring, does the temperature change?
|
No
|
|
|
While condensation or boiling is occurring, does the temperature change?
|
No
|
|
|
Where can you find the formulas with Hv or Hf and the values for Hv for Hf water?
|
On your reference pages
|
|
|
If you have a temperature change and you are trying to calculate the amount of heat needed to make that change, which formula do you use?
|
Q = m Cp ΔT
|
|
|
What does the symbol Cp mean?
|
Specific Heat
|
|
|
What does the symbol ΔT mean?
|
Change in temperature
|
|
|
Are the specific heats for all the states of water the same?
|
No, you must use a different Cp value for each state of matter ... found on the reference pages.
|
|
|
Which always take more energy if working with 1 g of substance-- melting or boiling?
|
boiling -- Hv values are higher than Hf values.
|
|
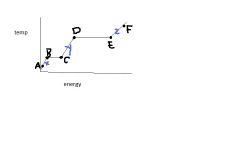
The energy between B & C is called ...
|
heat of fusion
|
|
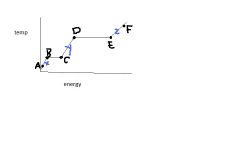
The energy between D & E is called....
|
heat of vaporization
|
|
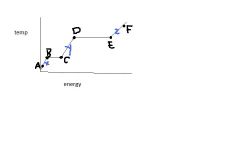
If this is water, what is the temperature change between C & D?
|
100 C
|
|
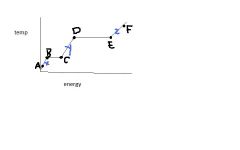
What are the states of matter for x, y, & z?
|
solid, liquid, gas -- in that order
|
|
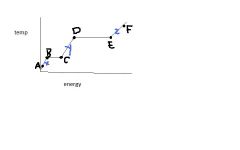
What phase change is occurring from B to C?... and from D to E?
|
melting
boiling |

