![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
23 Cards in this Set
- Front
- Back
|
The Law of Conservation of Energy |
Dictates that energy can be neither created nor destroyed, but that all thermal, chemical, potential, and kinetic energies are interconvertible. |
|
|
Isolated Systems |
No exchange of energy/matter with the environment. Bomb calorimetry creates a nearly isolated system. |
|
|
Closed Systems |
Can exchange energy but not matter with the environment. |
|
|
Open Systems |
Can exchange both energy and matter with the environment. |
|
|
Isothermal |
Temperature of system remains constant. |
|
|
Adiabatic |
No heat exchange occurs. |
|
|
Isobaric |
Pressure of system remains constant. |
|
|
Isovolumetric (isochoric) |
Volume remains constant. |
|
|
Heat |
The transfer of thermal energy from one subject to another. |
|
|
Endothermic |
Reactions that absorb thermal energy. |
|
|
Exothermic |
Reactions that release thermal energy. |
|
|
Constant-Volume and Constant-Pressure Calorimetry |
Used to indicate conditions under which the heat flow is measure. q = mc(delta T); where q is the heat absorbed or released in a given process, m is the mass, c is the specific heat, and (delta T) is the change in temperature. |
|
|
State Functions |
Include: Pressure, density, temperature, volume, enthalpy, internal energy, free energy, and entropy. |
|
|
Enthalpy (H) |
Used to express heat changes at constant pressure. |
|
|
Standard Heat of Formation |
The enthalpy change that would occur if one mole of a compound was formed directly from its elements in their standard states.
|
|
|
Standard Heat of Reaction |
Delta Hrxn = (Sum of delta Hf of Products) - (Sum of delta Hf of Reactants) |
|
|
Hess's Law |
States that enthalpies of reactions are additive; the reverse of any reaction has an enthalpy of the same magnitude as that of the forward reaction, but its sign is opposite. |
|
|
Bond Dissociation Energy |
An average of the energy required to break a particular type of bond in one mole of gaseous molecules. |
|
|
Bond Enthalpy (H) |
The standard heat of reaction can be calculated using the values of bond dissociation energies of particular bonds. Delta Hrxn = (Sum of Delta HBonds broken) -Sum of Delta HBonds formed) |
|
|
Entropy (S) |
The measure of the distribution of energy (randomness) throughout a system. (Delta Suniverse) = Delta Ssystem + Delta Ssurroundings |
|
|
Gibbs Free Energy (G) |
Delta G = Delta H - T(Delta S) If Delta G is negative, the reaction is spontaneous. If Delta G is positive, the reaction is non-spontaneous. If Delta G is zero, the system is in a state of equilibrium; thus, Delta H = T(Delta S) |
|
|
Gibbs Free Energy (G) |
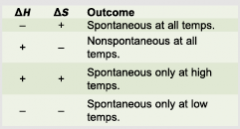
|
|
|
Reaction Quotient (Q) |
Once a reaction commence, the standard state conditions no longer hold. Q = [Products] / [Reactants] |

