![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
5 Cards in this Set
- Front
- Back
|
What is thermochemistry? |
Thermo chemistry is the heat involved in a chemical reaction. |
|
|
What is an Endothermic Reaction? |
Endothermic reaction has a positive dH and the products are less stable than the reactants. |
|
|
What is an Exothermic reaction? |
An exothermic reaction has a Negative dH and the products are more stable than the reactants. |
|
|
What is Enthalpy change of formation? |
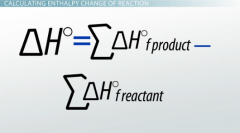
It is the energy change upon a substance of 1 mol from it basic from or its contituetent elements. We can use this formula to fund thee energy associated with the chemical formed. |
|
|
What is Enthaply change of combustion? |
Heat associated in combustion of 1 mol of a substance. |

