![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
53 Cards in this Set
- Front
- Back
|
Alcohols have the _____ functional group.
|
Hydroxyl
|
|
|
Ethers have the _____ functional group.
|
Alkoxy
|
|
|
Alcohols with more than one functional group are called:
|
Polyhydric.
|
|
|
Water forms _ hydrogen bonds.
|
2
|
|
|
Alcohols have what kind of intermolecular bond?
|
Hydrogen bonding. Permanent dipole - permanent dipole.
|
|
|
Secondary alcohol groups oxidise to form _____ groups.
|
Carbonyl. C double bond O. Forms a KETONE
|
|
|
Alcohols become _____ soluble as you increase chain length.
|
Less.
|
|
|
Carbonyl group structure:
|
Similar to an carboxylic acid, but in the centre of a chain; CH double bond O
|
|
|
Primary alcohols:
|
Have the OH on the terminal carbon of a carbon chain. The carbon is attached to one other.
|
|
|
Secondary alcohols:
|
Have the OH group bonded to a carbon somewhere in the middle of a chain. The carbon is attached to to others.
|
|
|
Tertiary alcohols:
|
Have the OH group bonded to a central carbon. The carbon is bonded to three others.
|
|
|
Primary alcohols are oxidised to:
|
Aldehydes. (Then carboxylic acids)
|
|
|
Aldehydes are oxidised to:
|
Carboxylic acids.
|
|
|
Aldehyde nomenclature:
|
Suffix -al
|
|
|
Tertiary alcohols are oxidised to:
|
Nothing. They do not oxidise.
|
|
|
Ketone nomenclature:
|
Suffix -one
|
|
|
Alcohols are usually oxidised by:
|
Heating with acidified solutions of potassium dichromate(VI) K2Cr2O2
|
|
|
Aldehydes and ketones both contain:
|
A carbonyl group.
|
|
|
The difference between an aldehyde and a ketone is:
|
The position of the functional group. Aldehydes have it on the end, ketones in the middle of a chain.
|
|
|
The dehydration of an alcohol produces:
|
An alkene and water
|
|
|
Dehydration of alcohols is what type of reaction?
|
Elimination.
|
|
|
Dehydration of alcohols is done by:
|
An alcohol vapour being passed over a hot alumina catalyst (Al2O3) at 300 degrees.
|
|
|
Are ethers less or more polar than alcohols?
|
Less.
|
|
|
Of comparable alcohols and ethers, which is more soluble in water and why?
|
Alcohols as they form hydrogen bonds more readily and dissolve better thus. More polar.
|
|
|
Alkene + halogen addition reaction:
|
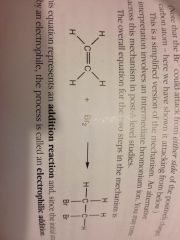
|
|
|
Alkene + halogen reaction:
|
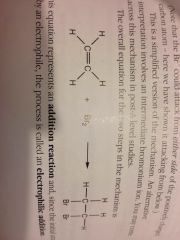
Electrophilic addition.
|
|
|
Alkenes enter ______ reactions because of:
|
Electrophilic addition. The region of negative charge a double bond represents.
|
|
|
One can easily test for an alkene by:
|
Shaking with bromine water. The bromine will decolourise.
|
|
|
Alkene electrophilic reactions include: (4)
|
Reactions with bromine and halogens, reactions with hydrogen bromide (and other hydrogen-halides) to form halogenoalkanes, reactions with water (to form alcohols, HYDRATION), reaction with hydrogen (to form alkanes, HYDROGENATION).
|
|
|
The addition of water to a alkene is a:
|
Electrophilic addition, specifically hydration.
|
|
|
Conditions for the hydration of an alkene:
|
phosphoric acid cat. adsorbed onto silica and steam - heated under pressure.
|
|
|
Conditions for the hydrogenation of an alkene:
|
Platinum catalyst (room temperature & pressure) or nickel (finely powdered, 150 degrees, five atmospheres.)
|
|
|
What kind of energy does infrared radiation confer to molecules?
|
Vibrational.
|
|
|
The leftmost, complex region of an IR spectrum is called:
|
The fingerprint region
|
|
|
What kind of energy does infrared radiation confer to molecules?
|
Vibrational.
|
|
|
Fibres:
|
Strong linear polymers that can form threads.
|
|
|
Chain length increase makes a polymer weaker or stronger?
|
Stronger.
|
|
|
Charges side groups make a polymer weaker or stronger?
|
Stronger.
|
|
|
Branching makes a polymer weaker or stronger?
|
Weaker
|
|
|
The leftmost, complex region of an IR spectrum is called:
|
The fingerprint region
|
|
|
What properties to plasticisers confer?
|
Flexibility, lowers melting point.
|
|
|
What properties do cross-links confer?
|
Greater strength and rigidity, higher melting points (often thermoset)
|
|
|
What properties do lots of crystalline regions confer to a polymer?
|
Lower flexibility, higher strength.
|
|
|
One can force crystalline regions to occur in fibres by:
|
Cold-drawing, forming a neck.
|
|
|
A co-polymer is formed by:
|
Repeating units of different types. (Different types of monomers)
|
|
|
Condensation polymerisation produces:
|
A polymer and some small molecules (often water or HCl)
|
|
|
Elastomers:
|
Soft, springy, flexible, stretchy (but return to original shape)
|
|
|
Plastics:
|
Experience plastic deformation, solid.
|
|
|
Stereoregularity makes a polymer weaker or stronger?
|
Stronger.
|
|
|
Cross-linking makes a polymer weaker or stronger?
|
Stronger.
|
|
|
Plasticisers makes a polymer weaker or stronger?
|
Weaker.
|
|
|
A thermoplastic polymer:
|
Has few or no cross links, and can be melted and reformed.
|
|
|
Thermoset polymers:
|
Have extensive cross-linking, cannot be melted, char instead.
|

