![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
13 Cards in this Set
- Front
- Back

Democritus |
Greek Philosopher,Thought if you cut something in half so many times it would not split he called it an atom. |
|

Aristotle |
Disagreed with Democritus |
|
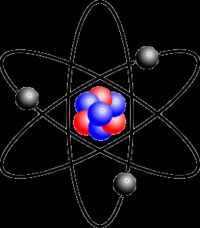
Atom |
Smallest particle that has the properties of an element |
|

John Dalton |
British chemist, experimented on Democritus |
|

J.J. Thomson |
Discovered electrons and that atoms were made up of even smaller particles |
|

Ernest Rutherford |
He found out that an atom has a nucleus and later he figured out that the nucleus is made up of smaller things |
|

Niels Bohr |
He a new created a theory that electrons behaved in a new way |
|
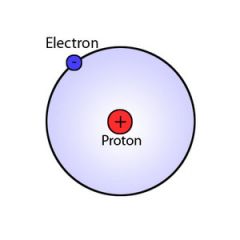
Electrons |
negatively charged particles |
|
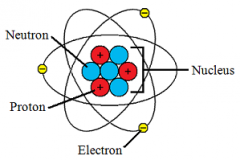
Nucleus |
A small,dense center that has a positive charge and is surrounded by moving electrons |
|
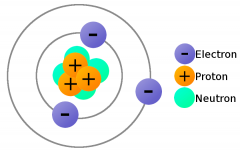
Protons |
Positively charged particles in the nucleus |
|
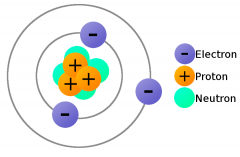
Neutrons |
Contains uncharged particles |
|

Electron cloud |
Electrons move within this area |
|

4 parts of the atomic theory |
Matter is made up of atoms, atoms cannot be created,divided, or destroyed |

