![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
15 Cards in this Set
- Front
- Back

Reactant |
Definition - a substance that takes part in and undergoes change during a reaction. My Definition - A substance that changes |
|

Product |
Definition - a substance produced during a natural, chemical, or manufacturing process: My Definition - The result of a chemical change |
|

Catalyst |
Defintion - a substance that increases the rate of a chemical reaction without itself undergoing any permanent chemical change. My Definition - |
|
Inhibitor |
Definition - a substance that slows down or prevents a particular chemical reaction or other process, or that reduces the activity of a particular reactant, catalyst, or enzyme. My Definition - A substance that reduces the activity of a reactant, catalyst, or enzyme. |
|
Rate of Rxn |
Definition - The reaction rate (rate of reaction) or speed of reaction for a reactant or product in a particular reaction is intuitively defined as how quickly or slowly a reaction takes place. My Definition - The speed of a reactant and product. |
|
Chemical Rxn |
Definition - Chemical reactions are chemical transformations. My Definition - Whenever a substance turns into another substance. |
|

Decomposition |
Definition - the state or process of rotting. My Definition - The state of decaying. |
|
Precipitation |
Definition - what happens in chemical reactions when a solid settles to the bottom of a solution My Definition - Whenever a gas turns into a liquid. |
|
Synthesis |
Definition - The act or process of forming a complex substance by combining or integrating two or more chemical entities, especially through a chemical reaction. My Definition - Creating substances by combining chemical substances. |
|
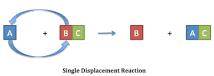
Single - Displacement |
Definition - A type of chemical reaction where an element reacts with a compound and takes the place of another element in that compound. My Definition - Whenever an element takes the place of another element. |
|

Double - Displacement |
Definition - a type of chemical reaction where two compounds react, and the positive ions and the negative ions of the two reactants switch places, forming two new compounds or products. My Definition - when the cations and anions switch between two reactants to form new products. |
|

Exothermic Rxn |
Definition - a process or chemical reaction characterized by or causing the liberation or release of heat. Combustion where heat is released is an example of an exothermic reaction. My Definition - Whenever heat is released. |
|
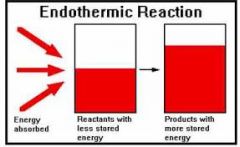
Endothermic Rxn |
Definition - in which the system absorbs energy from its surroundings; usually, but not always, in the form of heat.The opposite of an endothermic process is an exothermic process, one that releases, "gives out" energy in the form of heat. My Definition - Whenever something absorbs energy from the surrounding. |
|
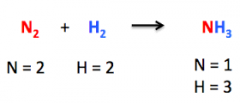
Chemical Equation |
Definition - A written representation of a chemical reaction, in which the symbols and amounts of the reactants are separated from those of the products by an equal sign, arrow, or a set of opposing arrows. My Definition - A written equation that shows the chemical reaction. |
|
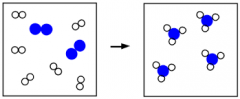
Conservation of Mass |
Definition - a principle stating that mass cannot be created or destroyed. My Definition - Whenever mass cannot be destroyed nor created. |

