![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
15 Cards in this Set
- Front
- Back
|
Substances initially present in a Blank blank that are consumed during the blank to make products. |
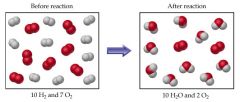
Reactant |
|
|
A substance that is formed when two or more chemicals react. When a chemical reaction takes place, a new substance is often created from the atoms or molecules of the original substances. There are often multiple blanks formed in a reaction. |
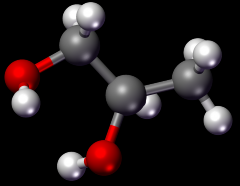
Product |
|
|
A substance that increases the rate of a chemical reaction without itself undergoing any permanent chemical change. |
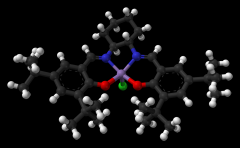
Catalyst |
|
|
A substance that reduces or suppresses the activity of another substance |
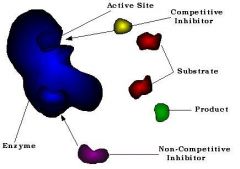
Inhibitor |
|
|
The decrease in the concentration of reactants per unit time or the increase in the concentration of product per unit time. |
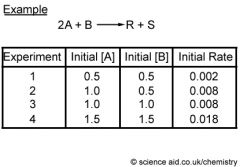
Rate of Reaction |
|
|
A process that involves rearrangement of the molecular or ionic structure of a substance, as opposed to a change in physical form or a nuclear reaction. |

Chemical Reaction |
|
|
A Blank reaction is a type of chemical reaction in which a single compound breaks down into two or more elements or new compounds. |
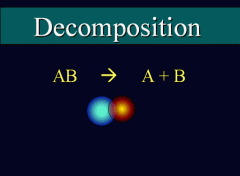
Decomposition Reaction |
|
|
A Blank reaction is a chemical reaction where one of the products is a precipitate. |

Precipitation Reaction |
|
|
A blank reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. |

Synthesis Reaction |
|
|
A blank reaction, also known as a blank reaction, is a type of chemical reaction where an element reacts with a compound and takes the place of another element in that compound. |
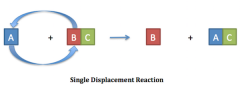
Single-Displacement Reaction |
|
|
A blank reaction, also known as blank reaction or metathesis, is a type of chemical reaction where two compounds react, and the positive ions and the negative ions of the two reactants switch places, forming two new compounds or products. |
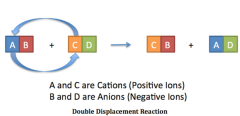
Double-Displacement Reaction |
|
|
An blank reaction is a chemical reaction that releases energy by light or heat. |

Exothermic Reaction |
|
|
The term blank reaction absorbs energy. |

Endothermic Reaction |
|
|
A blank equation is the symbolic representation of a chemical reaction in the form of symbols and formulae, wherein the reactant entities are given on the left-hand side and the product entities on the right-hand side. |
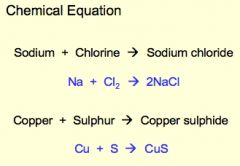
Chemical Equation |
|
|
A principle stating that blank cannot be created or destroyed. |
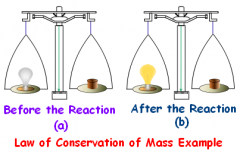
Conservation of Mass |

