![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
35 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
How is MHC diversity acheived?
What does Haplotype refer to? |
MHC genes do NOT rearrange - instead these are the most polymorphic genes in our body (you inherit a different allele from each parent)
Haplotype = the particular combination of HLA (MHC) alleles on a given Xsome 6 |
|
|
|
What immune receptor is capable of binding multiple different Ag's? (ie which receptor is permiscuous as opposed to being specific?
|
MHC can bind different Ag's and is not specific to one epitope like B & T cell receptors.
|
|
|
|
What is the ONLY way in which a T cell can recognize an Ag?
|
TCR can recognize Ag ONLY in the form of a peptide bound to an MHC
|
|
|
|
What does MHC restriction refer to?
|
Any give TCR is specific for both the Ag and the MHC (therefore specific to the Ag:MCH complex) and thus the T cell response is restricted by the MHC type
|
|
|
|
What type of cell will have MHC I? With this in mind what is an example of a cell that will not have MHC I?
|
All nucleated cells have MHC I. Since RBC's have no nucleus - they will NOT have MHC 1 !!
|
|
|
|
What is the role of the following proteins:
- Invariant chain - CLIP peptide - HLA-DM Are they involved with MHC I or II? |
- Invariant chain: protects MHC II peptide binding site in the ER
- CLIP peptide: once in a vesicle invariant chain is cleaved leaving only a CLIP fragment in binding site - HLA-DM: when Ag-containing vesicle arrives, this protein facilitates the release of CLIP so that Ag can bind MHC II |
|
|
|
What is the main difference between the assembly of MHC I and MHC II?
|
MHC I requires a peptide to bind to complete the folding of the molecule
(In MHC II assembly the binding site is blocked until the MHC is interacting with Ag) |
|
|
|
What is the role of the following proteins in MHC assembly:
- Proteasome - TAP - Calnexin/Calretiulin Are these involved with MHC I or II? |
- Proteasome: digests the intracellular Ag into peptides
- TAP: transports the peptides across the ER - Calnexin/Calretiulin: chaperone proteins that aid in the assembly of... MHC 1!!! |
|
|
|
The TCR is similar to one arm of the Ig molecule. That said, what are 2 main differences between T cell receptors and Ig's?
|
TCR's are signal tranducers - they only recognize Ag whereas Ig's both recognize & act as effector cells.
TCR's are Ag:MHC specific whereas Ig can bind epitopes on a wide range of intact molecules |
|
|
|
What is the order of gene segment rearrangements in T cell B-chains?
|
DJ rearrangement first, followed by a V - DJ rearrangement. Following this get inclusion of constant region
|
|
|
|
What successful outcome will allow the expression of CD4 and CD8 on the T cell surface?
|
Successful rearrangement of the B-chain and the expression of this B-chain with a surrogate alpha chain will allow CD4 & 8 to be expressed (also CD3)
|
|
|
|
Describe the process/steps of gene rearrangement in a T cell, staring from when the T cell migrates from the B marrow to the thymus:
* hint available * |
1. T cells starts by undergoing B G D rearrangements. If successful gamma delta - then it becomes this type of cell
2. If successful B rearrangement (D+J, --> DJ + V) then B is expressed on the surface of the cell with a surrogate alpha chain (** this stops B chain rearrangement! **) 3. Cells proliferate then alpha chain rearranges (VJ only - like light chains on B cells!) 4. Expression of successful aB chain halts a rearrangement now have a double positive (CD4 & 8) T cell! |
1. first rearrangement? 2 possible outcomes
2. If successful ____ then what does the cell do? What is the effect of doing this? 3. Now what do cells rearrange? 4. Expression of what does what? |
|
|
Where does +ve selection occur and what does it accomplish? Is the cell double +ve or double --ve at this point?
How does a T cell "pass" the +ve selection test? What happens if the T cell passes? Fails? |
+ve selection occurs in the thymic cortex, where epithelial cells express MHC I and II with self Ag's and are in close contact with DOUBLE +ve Tcells.
Tcells pass if they bind within 3-4days - the prize? Survival signals! If not binding the T cells are neglected and die by apoptosis. |
|
|
|
When does CD4 CD8 selection occur? And how does it happen?
|
When T cells are in the thymic Cortex, during +ve selection they are busily trying to bind to MHC + a self Ag. The class of MHC that they bind will determine their CD status - i.e. if they bind MHC II they will become CD4, if they bind an MHC I they will become CD8.
|
|
|
|
Which cells mediate --ve selection? Where does it occur? What is the mechanism? Is a T cell double + during --ve selection?
|
Professional APC's (ie Dendritic cells) mediate --ve selection. This occurs in the thymus, where tight binding of peptide:MHC induces the T cell to apoptose! (this is b/c these APC's are presenting self Ag's!)
|
|
|
|
The thymus has a "menu" of proteins to use for --ve selection (proteins from dendritic cells, from cells phagocytosed by Mphages & soluble proteins taken up from Ecellular fluids). But still, how does --ve selection prevent T cells from being self-reactive to peptides that are not found in the thymus?
|
--ve selection is able to prevent self-reactivity, even to non-thymic Ag's because of the gene AIRE (AutoImmune Regulator)
This gene is a Transcription factor that allows thymic endothelial cells to make proteins that are found elsewhere in the body (e.g. insulin). These tissue-specific peptides are bound by MHC I |
|
|
|
What (broadly speaking) are the 2 mechanisms of peripheral tolerance?
|
1. Anergy - b/c no co-stimulation
2. Suppression by Treg's |
|
|
|
What is the mechanism behind tissue transplant rejection?
(how does IS recognize donated tissue as foreign and what does it do?) |
Dendritic cells from the donated organ will migrate to secondary L-phoid tissues where they can present donor Ag to host Tcells.
The T cells get activated and go and attack the organ! |
|
|
|
What is the treatment for someone with APECED? (aka no AIRE gene?)
|
Bone Marrow Transplant to erradicate & replace all their self-reactive T cells
|
|
|
|
Why must bone marrow donors & recipients be "tightly tissue matched"? What exactly is being matched?
|
The MHC (aka HLA) must be very similar b/c the T cells of the donor are selected in the recipient thymus. In other words, donor T cells must recognize host MHC to survive +selection, but to be effective they must also recognize host MHC (on host APC's) otherwise they will not be able to recognize any Ag's.
|
|
|
|
In order to activate a T cell you need a costimulatory signal from the APC. What is the name of the T & APC molecule that provides this stimulation?
|
Tcell has CD28 <--> CD80 (B7) on the APC
|
|
|
|
In what 2 ways is the concept of costimulation used therapeutically?
|
Costimulation is inhibited in RA by Orencia which prevents the T activation that drives the chronic inflammation (binds B7/CD80 to block T CD2)
Adjuvants in vaccines induce costim (induce B7/CD80 production in the APC) |
|
|
|
What 2 signals are required to activate an Mphage? What makes this elegant?
|
When a Mphage presents it's Ag:MHC it has alterior motives! It may seem like it's activating the T cell, but really it's activating itself, because once the T cell is activated it will:
- secrete the Mphage activator IFN-gamma - present CD 40 Ligand which can bind the CD40 on the Mphage = Mphage activation! |
|
|
|
What cytokines are condusive to each of the following cell types:
TH1 TH2 |
TH1 cells: IL - 2 and IFN gamma
TH2 cells: IL-4 & IL-5 |
|
|
|
What determines the severity of a Leprosy infection?
|
Whether the immune system favors TH1 or TH2. Because leprosy is an intracellular infection, the TH1 response which activates Mphages will control it. The TH2 response though will produce useless Abs
|
|
|
|
T reg cells are important in peripheral tolerance. What 2 proteins do they express?
What 2 cytokines are secreted by Treg cells? |
Treg cells express FoxP3, a transcriptional repressor protein & MHC 2
They release TGF Beta and IL-10. The TGF-beta can induce naive T cells to to become Treg's. |
|
|
|
What happens if an APC provides a suboptimal costimulatory signal to a CD8 cell?
*hint* 2 ways of getting some assistance! |
Everyone's best friend the old CD4 helper cell comes to the rescue. When the CD4 helper cell is activated by the APC, it causes the APC to increase it's surface Ag and the IL-2 that it makes can also help the CD8 cell.
|
how does CD4 helper T cell help the situation?
|
|
|
What is beneficial about CD8 cells causing apoptsis?
|
Involution of cells (via apoptosis) prevents the release of pathogens into the circulation.
|
|
|
|
What are 2 ways CD8 cells can induce apoptosis?
|
1. Release their cytotoxic granules onto a cell (that's expressing an Ag to which they are specific!)
2. By binding their Fas Ligand onto the cell's Fas receptor |
|
|
|
How do Mphages recognize that something is bad?
What common pathway is involved? |
They have Toll-like receptors that are capable of recognizing 1 or > microbial molecular patterns.
All these receptors use a common pathway: - induce transcription factor NFk-beta which signals the Mphage to make & release inflammatory cytolines that recruit other cells |
|
|
|
Bacteria induce Mphages to release IL-6, where does IL-6 act and what 2 things does it cause to be secreted? (and what do these 2 things do)?
|
IL- 6 acts on Hepatocytes and causes them to release "acute phase proteins"
- C-reactive protein - Mannose-binding lectin Both of these act as opsonizers and can activate complement. |
|
|
|
What 3 things attract neutrophils to a site of infection?
What 2 things do they have to help them fight infection? |
Attractors:
- Mphages - inflammatory mediators - some bacterial Ag's - FC receptor so they can bind Ab's and phago things that have been opsonized - Complement receptors - induce MAC's |
|
|

Fill in the blanks of the this table
|

|
|
|
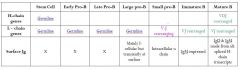
Complete this table
|

Good Job!
|
|
|
|
What is the only type of T cell that is not MHC restricted?
What is the implication of this on their functioning? |
gamma-delta, this makes them non-specific and their receptors instead recognize a group of Ag's that share common chemical features.
They have multiple functions in innate immunity. |
|

