![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
190 Cards in this Set
- Front
- Back
|
How are action potentials generated in a cell? |
The action potentials generated by a cell are all similar in size and duration, |
|
|
How is Vm determined in an action potential? |
Vm, can be determined by inserting a microelectrode in the cell. A voltmeter is used to measure the electrical potential difference between the tip of this intracellular microelectrode and another placed outside the cell. When the neuronal membrane |
|
|
What are some identifiable parts of the action potential? |
Rising phase: a rapid depolarization of the membrane thatcontinues until Vm reaches a peak value of about 40 mV. The part of the action potential where the inside of the neuron is positively charged with respect to the outside is called the overshoot. The falling phase of the action potential is a rapid repolarization until the membrane is actually more negative |
|
|
Chain of events to stimulate an action potential by pain |
(1) the thumbtack enters the skin, (2) the membrane of the nerve fibers in the skin is stretched, (3) Na-permeable channels open. Because of the large concentration gradient and the negative charge of the cytosol, Na |
|
|
Threshold |
The critical level of depolarization that must be crossed in order to trigger an action potential is called threshold. Action potentials are caused by depolarization of the membrane beyond threshold. |
|
|
Different ways depolarization can be caused |
-Entry of Na+ through specialized ion channels -In interneurons, depolarization is usually caused by Na entry through channels that are sensitive to neurotransmitters released by -neurons can be depolarized by injecting electrical current through a microelectrode
|
|
|
Intracellular v.s. extracellular recording |
Intracellular: The goal of intracellular recording is simple: to measure the potential difference between the tip of the intracellular electrode and another electrode placed in the solution bathing the neuron (continuous with the earth, Extracellular: we measure the potential difference between the tip of the recording electrode and ground. The electrode can be a fine glass capillary filled with a salt solution, but it is often simply a thin insulated metal wire. Normally, in the absence of neural activity, the |
|
|
Firing Frequency |
The cell generates action potentials at a rate of something like one per second, or 1 hertz (Hz). If we crank up the current a little bit more, however, we will find that the rate of action potential generation increases, say, to 50 impulses per second (50 Hz). Thus, the firing frequency of action potentials reflects the magnitude of the depolarizing current. |
|
|
What is the maximum firing frequency? |
Although firing frequency increases with the amount of depolarizing current, there is a limit to the rate at which a neuron can generate action |
|
|
Absolute Refractory Period |
Once an action potential is initiated, it is impossible to initiate another for 1 msec. This period of time is called the absolute refractory period. In addition, it can be relatively difficult to initiate another action potential for several |
|
|
Relative Refractory Period |
During this relative refractory period, the amount of current required to depolarize |
|
|
Three types of protein molecules in the ideal cell |
-Sodium-potassium pumps -potassium pumps -sodium channels |
|
|
What is the Nernst equation and how does it work? |
IK =gK(Vm-EK). 1. The net movement of K ions across the membrane is an electrical current. We can represent this current using the symbol IK. driving force on K is defined as the difference between the real membrane potential and the equilibrium potential, and it can be written as Vm -EK. |
|
|
How could we account for the falling phase of the action potential? |
Simply assume that sodium channels quickly close and the potassium channels remain open, so the dominant membrane ion permeability switches back from Na to K. Then K would flow out of the cell until the membrane potential again equals EK. Notice that if gK increased during the falling |
|
|
Voltage clamp |
The voltage clamp enabled Hodgkin and |
|
|
How did Hodgkin and Huxley determine transient changes in gNA? |
To account for the transient changes in gNa, Hodgkin and Huxley proposed the existence of sodium “gates” in the axonal membrane. They hypothesized that these gates are “activated”—opened—by depolarization above threshold and “inactivated”—closed and locked—when the membrane acquires a positive membrane potential. These gates are “deinactivated”— |
|
|
Voltage-gated sodium channel |
The protein forms a pore in the membrane that is highly selective to Na ions, and the pore is opened and closed by changes in the electrical potential of the membrane. |
|
|
Patch Clamp |
The patch-clamp method entails sealing the tip of an electrode to a very small patch of neuronal membrane. This patch then can |
|
|
Characteristic Pattern of Behaviour for Voltage-Gated Sodium Channels |
1. They open with little delay. A single channel does not an action potential make. The membrane of an axon may contain thousands of sodium channels per square micrometer (μm2), and the concerted action of all these channels is required to generate |
|
|
Steps of Patch Clamping |
Lower the fire-polished tip of a glass recording electrode, 1–5 μm in diameter, onto the membrane of the neuron (part a), and then apply suction through the electrode tip (part b). A tight seal forms between the walls of the electrode and the underlying patch of membrane. This “gigaohm” seal leaves the ions in the electrode only one path to take, through the channels in the underlying patch of membrane. If the electrode is then withdrawn from the cell, the membrane patch can be torn |
|
|
Generalized epilepsy with febrile seizures |
-This is a channelopathy due to single amino acid mutations in the extracellular regions of one -The seizures in this disorder occur in response to fever. They are usually confined to early childhood, between 3 months and 5 years of age. Although precisely how the seizures are triggered by an increase in brain temperature is not clear, among other effects, the mutations |
|
|
Tetrodotoxin (TTX) |
-Pufferfish toxin, which selectively blocks the sodium channel by binding to a specific site on the channel, and blocks all sodium-dependent action potentials -Fatal if ingested |
|
|
Saxitoxin |
Saxitoxin is produced produced by dinoflagellates of the genus Gonyaulax and concentrated in clams, mussels, and other shellfish that feed on these marine protozoa. Occasionally, the dinoflagellates bloom, causing what is known as a “red tide.” Eating shellfish at these times can be fatal, because of the unusually high concentration of the toxin. -This is a sodium channel toxin |
|
|
Batrachotoxin |
-Instead of blocking sodium channel toxins, it causes them to open inappropriately -Isolated from the skin of species of Colombian frog -Batrachotoxin causes the channels to open at |
|
|
Voltage-Gated Potassium Channels |
-Unlike sodium gates, potassium gates do not open immediately upon depolarization; it -The channel proteins consist of four separate polypeptide subunits that come together to form a pore between them.
|
|
|
Orthodromic Conduction |
Normally, action potentials conduct only in one direction; this is called orthodromic conduction. |
|
|
Antidromic Conduction |
Backward propagation, sometimes elicited experimentally, is called antidromic conduction.) Note that because the axonal membrane is excitable (capable of generating action potentials) along its entire length, |
|
|
Lidocaine |
Lidocaine and other local anesthetics prevent action potentials by binding to the voltage-gated sodium channels. The binding site for lidocaine has been identified as the S6 |
|
|
Saltatory conduction |
Myelin allows current to spread farther and faster between nodes, thus speeding action potential conduction. In myelinated axons, action |
|
|
Spike-Initiation Zone |
Another word for the spike initiation zone is "Axon Hillock" Its the area where the soma ends and the axon starts. Here, all EPSPs (Excitatory post-synaptic potentials) and IPSPs (inhibitory post-synaptic potentials) sum up in order to make an action potential either more or less likely to occur. Think of the axon hillock as a funnel where all the neuronal input gathers. |
|
|
Chemical Synapses |
-Comprises of most of the synapses in the body -The presynaptic and postsynaptic membranes at chemical synapses are separated by a synaptic cleft that is 20–50 nm wide, 10 times the width of -The axon terminal typically contains dozens of small membrane-enclosed spheres, each about 50 nm in diameter, called synaptic vesicles - |
|
|
Secretory Granules |
Many axon terminals also contain larger vesicles, each about 100 nm in diameter, called secretory granules. Secretory granules contain soluble protein that appears dark in the electron microscope, so they are sometimes called large, dense-core vesicles. |
|
|
Membrane Differentiations |
Dense accumulations of protein adjacent to and within the membranes on either side of the synaptic cleft are collectively called membrane differentiations. On the presynaptic side, proteins jutting into the cytoplasm of the terminal along the intracellular face of the membrane sometimes look like a field of tiny pyramids, called active zones. |
|
|
Active zones |
The pyramids, and the membrane associated |
|
|
Postsynaptic Density |
The protein thickly accumulated in and just under the postsynaptic membrane is called the postsynaptic density. The postsynaptic density contains the neurotransmitter receptors, which convert the intercellular chemical signal (i.e., neurotransmitter) into an intracellular signal (i.e., a change in membrane potential, or a chemical change) in the postsynaptic cell. |
|
|
CNS Synapses |
In the CNS, different types of synapse may be distinguished by which part of the neuron is postsynaptic to the axon terminal. If the postsynaptic membrane is on a dendrite, the synapse is said to be axodendritic.If the postsynaptic membrane is on the cell body, the synapse is said to be axosomatic. In some cases, the postsynaptic membrane is on another axon, and these synapses are called axoaxonic. When dendrites form synapses with each other, they are called dendrodendrites. |
|
|
Gray's type I versus gray's type II synapses |
Synapses in which the membrane differentiation on the postsynaptic side is thicker than that on the presynaptic side are called asymmetrical synapses, or Gray’s type I synapses; those in which the membrane differentiations are of similar thickness are called symmetrical synapses, or Gray’s type II synapses. Gray’s type I synapses are usually excitatory, while Gray’s type II synapses are |
|
|
Neuromuscular Junction |
Synaptic junctions also exist outside the |
|
|
Three chemical categories of neurotransmitters |
(1) amino acids, (2) amines, and (3) peptides |
|
|
Amino acids examples |
GABA, Glutamate, Glycine |
|
|
Amines |
Ach, dopamine, norepinephrine, histamine, epinephrine, 5-HT |
|
|
Peptides |
Cholecystokinin (CCK), Dynorphin, Enkephalins, N-acetylaspartylglutamate (NAAG), neuropeptide Y, somatostatin, substance P, thyrotropin-releasing hormone, vasoactive intestinal peptide (VIP) |
|
|
The amine acetylcholine (ACh) |
The amine acetylcholine (ACh) mediates fast synaptic transmission |
|
|
Voltage-Gated Calcium Channels |
-Similar to sodium channels, except they are for calcium -There is a large inward driving force on Ca2. Remember that the internal calcium ion concentration—[Ca2]i—at rest is very low, only 0.0002 mM; therefore, Ca2 will flood the cytoplasm of the axon terminal as long as the |
|
|
Transmitter-Gated Ion Channels |
Receptors known as transmittergated |
|
|
T and V SNARES |
Vesicles have “v-SNAREs,” and the outer membrane has “t-SNAREs” (for “target” |
|
|
excitatory postsynaptic |
A transient postsynaptic membrane depolarization caused by the presynaptic release of neurotransmitter is called an excitatory postsynaptic potential (EPSP). Synaptic activation of AChgated and glutamate-gated ion channels causes EPSPs. |
|
|
inhibitory |
Because it tends to bring the membrane potential away from threshold |
|
|
G-protein-coupled receptors steps |
1. Neurotransmitter molecules bind to receptor proteins embedded in the |
|
|
second messengers |
Effector proteins can be G-protein-gated ion channels in the membrane |
|
|
metabotropic receptors |
Because Gprotein- coupled receptors can trigger widespread metabolic effects, they are |
|
|
Autoreceptors |
Besides being a part of the postsynaptic density, neurotransmitter receptors are also commonly found in the membrane of the |
|
|
Cholinergic |
A term to describe cells that produce and release acetylcholine |
|
|
Noradrenergic |
The neurons that use the amine neurotransmitter norepinephrine (NE) are called noradrenergic. |
|
|
Glutamatergic and GABAergic synapses |
They use glutamate and GABA as synapses |
|
|
Peptidergic |
Peptidergic synapses use peptides |
|
|
Cholinergic system |
ACh and all the molecular machinery associated with it are collectively called the cholinergic system. |
|
|
Three criteria of a neurotransmitter |
1. The molecule must be synthesized and stored in the presynaptic neuron. |
|
|
Immunocytochemistry |
-One of the two methods along with in situ hybridization in the localization of transmitters and transmitter-synthesizing enzymes -Once the neurotransmitter candidate has been chemically purified, it is injected into the bloodstream of an animal, where it stimulates an immune response. Antibodies bind to specific sites on the foreign molecule. The best antibodies for immunocytochemistry bind very tightly to the transmitter of interest, and |
|
|
In situ hybridization |
-One of the two methods along with immunocytochemistry in the localization of transmitters and transmitter-synthesizing enzymes. -If the sequence of nucleic acids in a strand of mRNA is known, it is possible to construct in the lab a complementary strand that will stick, like a strip of Velcro, to the mRNA molecule. The complementary strand is called a probe, -In this method the probes are usually labelled by making them hyperactive |
|
|
Autoradiography |
Hybridized probes are detected by laying the brain tissue on a sheet of special film that is sensitive to radioactive emissions. After exposure to the tissue, the film is developed |
|
|
How do in vitro brain slices work? |
-Since most of the synapses in the CNS (unlike in the PNS) are intermingled, they are difficult to study, so it's hard to stimulate a single population of synapses with a single neurotransmitter -To stimulate release, the slices are bathed in a solution containing a high K concentration. This treatment causes a large membrane depolarization, thereby stimulating transmitter release from the axon terminals in the tissue. Because transmitter release requires the entry of Ca2 into the axon terminal, it must also be shown that the release of the neurotransmitter candidate from the tissue slice after depolarization occurs only when Ca2 ions are present in the bathing solution. |
|
|
Microionophoresis |
-A method used to study synaptic mimicry in postsynaptic cells -This method enables a researcher to apply drugs or neurotransmitter candidates in very small amounts to the surface of neurons. The responses generated by the drug can be compared to those generated by synaptic stimulation. |
|
|
Receptor Subtype |
As a rule, no two neurotransmitters bind to the same receptor; however, one neurotransmitter can bind to many different receptors. Each |
|
|
Two ACh receptor subtypes |
Nicotinic ACh receptors in skeletal muscle and muscarinic ACh receptors in the heart. Nicotinic and muscarinic receptors also exist in the brain. |
|
|
Three receptor subtypes of glutamate receptors |
AMPA, NMDA, kainate receptors. The neurotransmitter glutamate activates all |
|
|
Opiates |
Opiates are a broad class of drugs that are both medically important and commonly abused. Their effects include pain relief, euphoria, |
|
|
Endorphins |
Endorphins are endogenous opioid inhibitory neuropeptides. They are produced by the central nervous system and pituitary gland. The term implies a pharmacological activity (analogous to the activity of the corticosteroid category of biochemicals) as opposed to a specific chemical formulation. |
|
|
Enkephalins |
-A type of opiate neurotransmitters -An enkephalin is a pentapeptide involved in regulating nociception in the body. The enkephalins are termed endogenous ligands, as they are internally derived and bind to the body's opioid receptors. |
|
|
Ligand-binding method |
Any chemical compound that binds to a specific site on a receptor is called a ligand for that receptor. The technique of studying receptors using radioactively labeled ligands is called the ligand-binding method. Ligand-binding methods have been enormously |
|
|
Dale’s principle |
The idea that a neuron has only one neurotransmitter is often called Dale’s principle. Many peptide-containing neurons violate Dale’s principle because these cells usually release more than one neurotransmitter: an amino acid or amine and a peptide. |
|
|
Co-transmitters |
When two or more transmitters are released from one nerve terminal, they are called co-transmitters. Co-transmitters violate Dale's principle |
|
|
Choline acetyltransferase (ChAT) |
The enzyme involved in synthesis of ACh. Only cholinergic neurons contain ChAT, so this enzyme is a good marker for cells that use ACh as a neurotransmitter. |
|
|
ACh Transporter |
ChAT synthesizes ACh in the cytosol of the axon terminal, and the neurotransmitter is concentrated in synaptic vesicles by the actions of an ACh transporter. |
|
|
Rate limited step of ACh synthesis |
Choline is taken up by the cholinergic axon terminals via a specific transporter. Because the availability of choline limits how much ACh can be synthesized in the axon terminal, the transport of choline into the neuron is said to be the rate-limiting step in ACh synthesis. |
|
|
Acetylcholinesterase (AChE) |
AChE degrades ACh into choline and acetic acid. AChE is secreted into the synaptic cleft and is associated with cholinergic axon terminal membranes. However, AChE is also manufactured by some noncholinergic neurons, so this enzyme is not as useful a marker for cholinergic synapses as ChAT. Much of the resulting choline is taken up by |
|
|
Catecholamines |
-The amino acid tyrosine is the precursor for three different amine neurotransmitters that contain a chemical structure called a catechol. The catecholamine neurotransmitters are dopamine (DA), norepinephrine (NE), and epinephrine, also called adrenaline. Catecholaminergic neurons are found in regions of the nervous system involved |
|
|
Tyrosine hydroxylase |
This catalyzes the first step in catecholamine synthesis, the conversion of tyrosine to a compound called dopa. All catecholamines contain this. |
|
|
Two general types of neurotransmitter transporter |
One type, the neuronal membrane transporter, shuttles transmitter from the extracellular fluid, including the synaptic cleft, and concentrates it up to 10,000 times higher within the cytosol of the presynaptic terminal. A second type, the vesicular transporter, |
|
|
Example of end-product inhibition in catecholamines |
Decreased catecholamine release by the |
|
|
A strategy to treat Parkinson's disease using catecholamines or precursors |
One strategy for treating Parkinson’s disease is the administration of dopa, which causes an increase in DA synthesis in the surviving neurons, increasing the amount of DA available for release. |
|
|
Dopamine-hydroxylase (DBH), |
An enzyme that converts dopamine into norepinephrine. It is interesting to note |
|
|
Phentolamine N-methyltransferase |
-Adrenergic neurons contain this enzyme -It converts norepinephrine into epinephrine -Curiously, PNMT is in the cytosol of adrenergic axon terminals. Thus, NE must first be synthesized in the vesicles, released into the cytosol for conversion into epinephrine, and then the epinephrine must again be transported into vesicles for release. |
|
|
Monoamine oxidase (MAO) |
-This helps to degrade catecholamines -Once inside, the axon terminal, the catecholamines may be reloaded into synaptic vesicles for reuse, or they may be enzymatically destroyed by the action of monoamine oxidase (MAO), an enzyme found on the outer membrane of mitochondria. |
|
|
Synthesis of serotonin |
Tryptophan is converted first into an intermediary called 5-HTP (5-hydroxytryptophan) by the enzyme tryptophan hydroxylase. The 5-HTP is then converted to 5-HT by the enzyme 5-HTP decarboxylase. Serotonin synthesis appears to be limited by the availability of tryptophan |
|
|
Serotonin Reuptake |
Following release from the axon terminal, 5-HT is removed from the synaptic cleft by the action of a specific transporter. The process of serotonin reuptake, like catecholamine reuptake, is sensitive to a number of different |
|
|
How are glutamate and GABA synthesized and broken down? |
Glutamatic acid decarboxylase (GAD) is used to turn glutamate into GABA. The synaptic actions of the amino acid neurotransmitters are terminated by selective uptake into the presynaptic terminals and glia, once again via |
|
|
Retrograde messengers |
-Endocannabinoids (endogenous cannabinoids) are an example of this -Retrograde signalling is communication from the "post" to "pre" direction -Retrograde messengers serve as a kind of feedback system to regulate the conventional forms of synaptic transmission, which of |
|
|
Two types of cannabinoid receptors |
Two types of cannabinoid receptors are now known: CB1 receptors are in the brain, and CB2 receptors are mainly in immune tissues elsewhere in the body. |
|
|
Several unusual qualities about endocannabinoids |
1. They are not packaged in vesicles like most other neurotransmitters; instead, they are manufactured rapidly and on-demand. |
|
|
Nitric Oxide |
NO may be another example of a retrograde messenger. Because NO is small and membrane permeable, similar to endocannabinoids, it can diffuse much more freely than most other transmitter molecules, even penetrating through one cell to affect another beyond it. Its influence may spread throughout a small region of local tissue, rather than being confined to the site of the cells that released them. On the other |
|
|
Transmitter-Gated Channels |
-11 nm long -It is a pentamer, an amalgam of five protein subunits arranged like the staves of a barrel to form a single pore through the membrane |
|
|
Properties of amino acid-gated channels |
-The pharmacology of their binding sites describes which transmitters affect them and how drugs interact with them. |
|
|
Glutamate-Gated Channels |
Three glutamate receptor subtypes bear the names of their selective agonists: AMPA, NMDA, and kainate. Each of these is a glutamate-gated ion channel. The AMPAgated AMPA-gated channels are permeable to both Na and K, and most of them are not permeable to Ca2. NMDA-gated channels are permeable to Ca2, and it is voltage dependent.
|
|
|
The Ubiquitous G-Proteins |
G-proteins all have the same basic mode of operation: 5. The G-alpha and G-beta-gamma subunits come back together, allowing the cycle to begin |
|
|
The Shortcut Pathway of G-protein coupled effector systems |
(a) G-proteins in heart muscle are activated by ACh binding to muscarinic receptors. (b) The activated G subunit directly gates a potassium channel. |
|
|
Second Messenger Cascade |
The whole process that couples the neurotransmitter, via multiple steps, to activation |
|
|
Divergence |
The ability of one transmitter to activate more than one subtype of receptor, and cause more than one type of postsynaptic response, is called divergence. |
|
|
Convergence |
Neurotransmitters can also exhibit convergence of effects. Multiple transmitters, |
|
|
Anatomical references in the brain |
The anatomical reference, pointing toward the rat’s nose is known as anterior or rostral. The direction pointing toward the rat’s tail is posterior or caudal. The direction pointing up is dorsal, and the direction pointing down is ventral. |
|
|
Bilateral symmetry |
A characteristic of the brain which means it can be divided into two equal halves. The invisible line running down the middle is called the midline, and structures near the midline are called medial. Structures farther away from the midline are called lateral. If two structures are on the same side it is called ipsilateral and structures on opposites sides are called contralateral. |
|
|
Three anatomical planes of section |
Midsagittal plane: splits the brain in left and right halves. Sections parallel to the midsagittal plane are in the sagittal plane. The horizontal plane is parallel to the ground and splits the brain in dorsal and ventral sections. The coronal plane is perpendicular to the ground and it splits the anterior and posterior of the brain. |
|
|
The Cerebrum |
-Largest and rostal-most part of the brain -There are two cerebral hemispheres down the middle, separated by the deep sagittal fissure -The right hemisphere receives sensations and movements from the left side of the body and the left hemisphere receives sensations and movements from the left side of the body |
|
|
The Cerebellum |
-Lies behind the cerebrum, like a little brain -Contains as many neurons as both cerebral hemispheres combined -It is a primarily motor control center with extentions in the cerebrum and spinal cord -The left side of the cerebellum corresponds to the left side of the body and the right side corresponds to the right side of the body
|
|
|
The Brain Stem |
-Forms a stalk from which the cerebrum and cerebellum sprout -Serves to relay information from cerebrum to spinal cord and cerebellum, and vice versa -The the brain stem is also the site where |
|
|
The Spinal Cord |
-The spinal cord is encased in the bony vertebral column and is attached to the brain stem -It is the major conduit of information from the skin, joints, and muscles of the body to the brain, and vice versa -A transection of the spinal cord results in anesthesia (lack of feeling) and paralysis of muscles in parts of the body caudal to the cut. This paralysis does not mean that they cannot function, but that they cannot be controlled by the brain -The spinal cord communicates with the body via spinal nerves, which are part of the PNS -Each spinal nerve attaches to the spinal cord by means of two branches, the dorsal root and the ventral root |
|
|
The Somatic PNS |
-All the spinal nerves that innervate the skin, the joints, and the muscles that are under voluntary control are part of the somatic PNS. -The somatic sensory axons, which innervate and collect information from the skin, muscles, and joints, enter the spinal cord via the dorsal roots. The cell bodies of these neurons lie outside the spinal cord in clusters called dorsal root ganglia. There is a dorsal root ganglion for each spinal nerve |
|
|
The Visceral PNS (also called Autonomic Nervous System) |
-The visceral PNS consists of the neurons that |
|
|
Afferent and Efferent Neurons |
Afferent neurons carry information towards a point and efferent neurons carry information away from a point. |
|
|
Cranial nerves |
-There are 12 cranial nerves that arise from the brain stem and innervate (mostly) the head -Many cranial nerves contain a complex mixture of axons that perform different functions -They are located in the CNS, and both divisions of the PNS |
|
|
The Meninges |
-The three membranes protecting the brain from the bones in the skull -The outer most membrane is the dura mater, has a leather-like consistency and forms a tough inelastic bag -The arachnoid membrane is right under the dura mater, and it has an appearance and consistency like a spider web. If the blood vessels passing through the dura are ruptured, blood can collect here and form what is called a subdural hematoma -The pia mater is under the arachnoid membrane, and it is a thin membrane that adheres closely to the brain -Along the pia run many blood vessels that ultimately dive into the substance of the underlying brain. The pia is separated from the arachnoid by a fluid-filled space. This subarachnoid space is filled with salty clear liquid called cerebrospinal fluid (CSF). Thus, in
|
|
|
The Ventricular System |
-The fluid-filled caverns and canals inside the brain constitute the ventricular system -The fluid in this system is the cerebrospinal fluid, which is the same as the fluid in the subarachnoid space, which is produced by choroid plexus in the ventricles -The CSF flows from the paired ventricles of the -In the subarachnoid space, CSF is absorbed by the blood vessels at special structures called arachnoid villi. If the normal flow of CSF is disrupted, brain damage can result |
|
|
Computed Tomography |
The goal of CT is to generate an image of a slice of brain. To accomplish this, an X-ray source is rotated around the head within the plane of the desired cross section. On the other side of the head, in the trajectory of the X-ray beam, are |
|
|
Magnetic Resonance Imaging (MRI) |
-Yields a more detailed map of the brain than CT -it doesn't require X-irradiation, and images of brain slices can be made in any plane desired -MRI uses information about how hydrogen atoms in the brain respond to perturbations of a strong magnetic field. The electromagnetic signals emitted by the atoms are detected by an array of sensors around the head and fed to a powerful computer that constructs a map of the brain. |
|
|
Functional Brain Imaging |
-Two types are PET (Positron emission tomography) and functional magnetic resonance imaging (fMRI) -The basic principle is simple. Neurons that are active demand more glucose and oxygen. The brain vasculature responds to neural activity by directing more blood to the active regions. Thus, by detecting changes in blood flow, PET |
|
|
Gray Matter |
A generic term for a collection of neuronal cell bodies in the CNS. When a freshly dissected brain is cut open, neurons appear gray. |
|
|
Cortex |
Any collection of neurons that form a thin sheet, usually at the brain’s surface. Cortex is Latin for |
|
|
Nucleus |
A clearly distinguishable mass of neurons, usually deep in the brain (not to be confused with the |
|
|
Substantia |
A group of related neurons deep within the brain, but usually with less distinct borders than those of nuclei. Example: substantia nigra (from the Latin for “black substance”), a brain stem cell group involved in the control of voluntary movement. |
|
|
Locus |
A small, well-defined group of cells. Example: locus coeruleus (Latin for “blue spot”), a brain stem cell group involved in the control of wakefulness and behavioral arousal. |
|
|
Ganglion |
A collection of neurons in the PNS. Ganglion is from the Greek for “knot.” Example: the dorsal root ganglia, which contain the cells bodies of sensory axons entering the spinal cord via the dorsal roots. Only one cell group in the CNS goes by this name: the basal ganglia, which are structures lying deep within the cerebrum that control movement. |
|
|
Endoderm |
The endoderm ultimately gives rise to the lining of many of the internal organs (viscera) |
|
|
Mesoderm |
From the mesoderm arise the bones of the skeleton and the muscles. |
|
|
Ectoderm |
The nervous system and the skin derive entirely from the ectoderm. The ectoderm also gives rise to the neural plate. |
|
|
Nerve |
A bundle of axons in the PNS. Only one collection of CNS axons is called a nerve: the optic nerve. |
|
|
White matter |
A generic term for a collection of CNS axons. When a freshly dissected brain is cut open, axons |
|
|
Tract |
A collection of CNS axons having a common site of origin and a common destination. Example: |
|
|
Bundle |
A collection of axons that run together but do not necessarily have the same origin and destination. |
|
|
Capsule |
A collection of axons that connect the cerebrum with the brain stem. Example: internal capsule, which connects the brain stem with the cerebral cortex. |
|
|
Commissure |
Any collection of axons that connect one side of the brain with the other side. |
|
|
Lemniscus |
A tract that meanders through the brain like a ribbon. Example: medial lemniscus, which brings touch information from the spinal cord through the brain stem. |
|
|
Formation of the neural tube and neural crest |
Formation of the neural tube and neural crest. These schematic illustrations follow |
|
|
Neurulation |
The process by which the neural plate becomes the neural tube is called neurulation. Neurulation occurs very early in embryonic development, |
|
|
Differentiation |
The process by which structures become more complex and functionally specialized during development is called differentiation. |
|
|
Three Primary Brain Vesicles |
-The entire brain is comprised of three primary vesicles of the neural tube -The prosencephalon is also called the forebrain. Behind the prosencephalon lies another vesicle called the mesencephalon, or midbrain. Caudal to this is the third primary vesicle, the rhombencephalon, or hindbrain. The rhombencephalon connects with the caudal neural tube, which gives rise to the |
|
|
Differentiation of the forebrain |
-Secondary vesicles sprout off both sides of the proencephalon -The unpaired structure after vesicles have sprouted off is called diencephalon -Optic stalks are formed, and they become the optic nerves and the two retinas in adults
|
|
|
Differentiation of the Telencephalon |
The telencephalon continues to develop |
|
|
Fluid-filled spaces within the cerebral hemispheres |
The fluid-filled spaces within the cerebral hemispheres are called the lateral ventricles, and the space at the center of the diencephalon is |
|
|
Two types of gray matter in the telencephalon |
These neurons form two different types of gray matter in the telencephalon: the cerebral cortex and the basal telencephalon. |
|
|
What does the diencephalon differentiate into? |
The diencephalon differentiates into |
|
|
Three major white matter systems in the developing forebrain |
The cortical white matter contains all the axons that run to and from the neurons in the |
|
|
Cerebral Cortex |
The cortex is the brain structure that has expanded the most over the course of human evolution. Cortical neurons receive sensory information, form perceptions of the outside world, and command voluntary movements. |
|
|
The thalamus: gateway to the cerebral |
The sensory pathways from the eye, ear, and skin all relay in the thalamus before terminating in the cerebral cortex. The arrows indicate the direction of information flow. |
|
|
Optic nerve |
The optic nerve connects the eye to the brain. The optic nerve carries the impulses formed by the retina, the nerve layer that lines the back of the eye and senses light and creates impulses. These impulses are dispatched through the optic nerve to the brain, which interprets them as images. |
|
|
Differentiation of the midbrain |
-It differentiates little during subsequent brain development -The dorsal surface of the mesencephalic vesicle becomes a structure called the tectum. The floor of the midbrain becomes the tegmentum. The CSF-filled space in between constricts into a narrow channel called the cerebral aqueduct. The aqueduct connects rostrally with the third ventricle of the |
|
|
Two structures of the tectum in the midbrain |
-The superior colliculus receives direct input from the eye, so it is also called the optic tectum. One function of the optic tectum is to control eye movements, which it does via synaptic connections with the motor neurons that innervate the eye muscles. -The inferior colliculus also receives sensory information, but from the ear instead of the eye. The inferior colliculus serves as an important relay station for auditory information en route to the thalamus. |
|
|
The Tegmentum In the Midbrain |
The tegmentum is one of the most colorful regions of the brain because it contains both the substantia nigra (the black substance) and the red nucleus. These two cell groups are involved in the control of voluntary movement. Other cell groups scattered in the midbrain have axons that project widely throughout much of the CNS and function to regulate consciousness, mood, pleasure, and pain. |
|
|
Differentiation of the Hindbrain |
The hindbrain differentiates into three important structures: the cerebellum, The medulla contains the cardiac, respiratory, vomiting and vasomotorcenters and therefore deals with the autonomic (involuntary) functions of breathing, heart rate and blood pressure. |
|
|
Differentiation of the spinal cord |
The spinal canal (or vertebral canal or spinal cavity) is the space in vertebrae through which the spinal cord passes.Cut in cross section, the gray matter of the spinal cord (where the neurons |
|
|
Lateral ventricles |
Related brain structures: Cerebral cortex
|
|
|
Third ventricle |
Related brain structures: Thalamus |
|
|
Cerebral aqueduct |
Related brain structures: Tectum |
|
|
Fourth Ventricle |
Related brain areas: Cerebellum |
|
|
Sulci and gyri |
The many convolutions on the surface of the human cerebrum: The grooves in the surface of the cerebrum are called sulci (singular: sulcus), and the bumps are called gyri (singular: gyrus). Remember, the thin sheet of neurons that lies just under the surface of the cerebrum is the cerebral cortex. |
|
|
Temporal lobe |
The tip of the “horn” lies right under |
|
|
Frontal lobe |
The portion of the cerebrum lying just |
|
|
Central Sulcus |
The deep central sulcus marks the posterior border of the frontal lobe, caudal to which lies the parietal lobe, under the parietal bone. Caudal to that, at the back of the cerebrum under the occipital bone, lies the occipital lobe |
|
|
Types of Cerebral Cortex |
First, the cell bodies of cortical neurons |
|
|
Cytoarchitectural map of the neocortex |
-Constructed by German neuroanatomist Korbinian Brodmann -In this map, each area of cortex having |
|
|
Three types of cortex that the neocortex exists in |
The first type consists of primary sensory areas, which are first to receive signals from the ascending sensory pathways. For example, area 17 is designated as primary visual cortex, |
|
|
Somatic Sensation |
Somatic sensation enables our body to feel, to ache, to chill, and to know what its parts are doing. It is sensitive to many kinds of stimuli: the pressure of objects against the skin, the position of joints and muscles, distension of the bladder, and the temperature of the limbs and of the brain itself. When stimuli become so |
|
|
Two ways somatic sensory system is different from other sensory systems |
1. Its receptors are distributed throughout the body instead of being concentrated at small, specialized locations. 2. It responds to many different type of stimuli instead of just one. |
|
|
Two major types of skin |
The two major types of skin are called hairy and glabrous (hairless), as exemplified by the backs and palms of your hands. |
|
|
How sensitive is skin? |
Skin is sensitive enough that a raised dot |
|
|
Mechanoreceptors |
-They are sensory receptors in the somatic sensory system which are sensitive to physical distortion such as bending or stretching. -Present throughout the body, they monitor contact with the skin, as well as pressure in the heart and blood vessels, stretching of the digestive organs and urinary bladder, and force against the teeth. At the heart of each mechanoreceptor are unmyelinated axon branches. These axons have mechanosensitive ion channels; their gating depends on stretching, or changes in tension, of the surrounding membrane. |
|
|
Pacinian corpuscle |
-A mechanoreceptor that is in the skin, the largest and best studied receptor -It can be as long as 2 mm and almost 1 mm in diameter—big enough to be seen with your -functioning as a sensory receptor of pressure and vibration -rapidly adapting |
|
|
Ruffini's endings |
Found in both hairy and glabrous skin, are slightly smaller than Pacinian corpuscles. Slowly adapting |
|
|
Meissner's corpuscles |
Meissner’s corpuscles are about one tenth -Rapidly adapting |
|
|
Merkel's disks |
Located within the epidermis, Merkel’s disks each consist of a nerve terminal and a flattened, -Slowly adapting |
|
|
Krause end bulbs |
In Krause end bulbs, which lie in the border regions of dry skin and mucous membrane (around the lips and genitals, for example), the nerve terminals look like knotted balls of string. |
|
|
What vibrations are pacinian and meissner corpuscles sensitive to? |
Pacinian corpuscles are most sensitive to vibrations of about 200–300 Hz, while Meissner’s corpuscles respond best around 50 Hz. |
|
|
Two Point Discrimination |
Two-point discrimination varies at least twentyfold across the body. Fingertips have the highest resolution. The dots of Braille are 1 mm high and 2.5 mm apart; up to six dots make a letter. An experienced Braille reader can scan an index finger across a page of raised dots and read about 600 letters per minute, which is roughly as fast as someone reading aloud. |
|
|
Primary afferent neuron |
Axons bringing information from the somatic sensory receptors to the spinal cord or brain stem are the primary afferent axons of the somatic sensory system. The primary afferent axons enter the spinal cord through the dorsal roots; their cell bodies lie in the dorsal root ganglia. Primary afferent axons have widely varying diameters, and their size correlates with the type of sensory receptor to which they are attached. |
|
|
How are primary afferent neurons named according to their sizes? |
A-alpha: Group 1, have a diameter of 13-20 um, speed of 80-120 m/sec, their sensory receptors are proprioceptors of skeletal muscle A-beta: Group 2, have diameter of 6-12 um, speed of 35-75, and their sensory receptors are mechanoreceptors of skin.= A-gamma: Group 3, diameter 1-5 um, speed 5-30 m/sec, sensory receptors pain and temperature C: Group 4, diameter 0.2-1.5, speed 0.5-2 m/sec, sensory receptors temperature, pain, itch |
|
|
Spinal segments |
-There are 30 of them in the spine -They are divided into 4 groups, namely cervical which begin at the neck (and comprise of 8 sections), thoracic around mid body (next 12), lumbar cord (next 5, towards the end of the spinal cord), and sacral cord (end of spinal cord, last 5) -Spinal nerves are named for the level of the spinal cord from which they exit and are numbered in order from rostral to caudal. |
|
|
Dermatosomes |
The segmental organization of spinal nerves and the sensory innervation of the skin are related. The area of skin innervated by the right and left dorsal roots of a single spinal segment is called a dermatome; thus, there is a |
|
|
How do you lose sensation in one dermatosome? |
When a dorsal root is cut, the corresponding dermatome on that side of the body does not lose all sensation. The residual somatic sensation is explained by the fact that the adjacent dorsal roots innervate overlapping areas. To lose all sensation in one dermatome, therefore, three adjacent dorsal roots must be cut. |
|
|
Cauda equina |
The bundles of spinal nerves streaming |
|
|
Lumbar puncture |
The cauda equina courses down the spinal |
|
|
What is each half of the gray matter in the spinal cord divided into? |
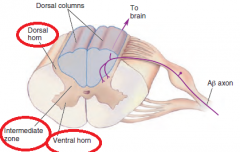
|
|
|
Second-order sensory neurons |
The neurons that receive sensory input from primary afferents are called second-order sensory neurons. Most of the second-order sensory neurons of the spinal cord lie within the dorsal horns. |
|
|
The dorsal column–medial lemniscal pathway |
The ascending branch of the large sensory axons (A-beta) enters the ipsilateral dorsal column of the spinal cord, the white matter tract medial to the dorsal horn. The dorsal columns carry information about tactile sensation and limb position toward the brain. The axons of the dorsal column terminate in the dorsal column nuclei, which lie at the junction of the spinal cord and medulla. At this point in the pathway, information is still represented ipsilaterally; |
|
|
Contrast enhancement |
The amplification of differences in |
|
|
Lateral inhibition |
Where neighboring cells inhibit one another. |
|
|
Trigeminal nerves |
The trigeminal nerve (the fifth cranial nerve, or simply CN V) is a nerve responsible for sensation in the face and motor functions such as biting and chewing. There are twin |
|
|
Area 3b |
Area 3b is the primary somatic sensory cortex because (1) it receives dense inputs from the VP nucleus of the thalamus; (2) its neurons are |
|
|
Somatotopy |
The mapping of the body’s surface sensations onto a structure in the brain is called somatotopy. |
|
|
Retinotopy |
We have seen previously that the brain has maps of other sensory surfaces, such as the light-sensitive retina in the eye (retinotopy) |
|
|
Tonotopy |
The brain has maps of sensory surfaces, such as the frequency sensitive cochlea in the inner ear (tonotopy). |
|
|
Homunculus |
A somatotopic map is sometimes called a homunculus (from the Latin diminutive of “man”; the little man in the brain). |

