![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
148 Cards in this Set
- Front
- Back
|
Everything preganglionic is ____.
|
ACh.
|
|
|
Postganglionic neurotransmitters (NT) are ____. What is the exception?
|
Norepinephrine (NE). The exception is sweat glands which is ACh.
|
|
|
What is the NTs for the postganglionic SNS, PNS and somatic NS?
|
SNS: NE (adrenergic)
PNS: muscarinic ACh Somatic NS: nicotinic ACh |
|
|
What receptors does norepinephrine agonize?
|
Postsynaptic a1,a2,b1 and b2.
|
|
|
From where and when is epinephrine released? Where does this NT act?
|
Released from adrenal gland in times of stress and acts on same receptors as NE.
|
|
|
How is NE synthesized in the presynaptic neuron?
|
Phenylalanine to tyrosine, then hydroxylated to dopa.
Dopa then decaboxylated to dopamine. Dopamine to dopamine B hydroxylase, then to NE (in synaptic vessels) |
|
|
Describe step 1 and 2 of the process of neurotransmission.
|
1. NT synthesized from chemical precursors.
2. Packed into vesicles in the presynaptic terminal where chromaffin cells store catacholamine in the adrenal medulla. |
|
|
Where is epinephrine synthesized and why?
|
In the adrenal medulla d/t the high concentration of N-methyltransferase.
|
|
|
What is step 3 and 4 of the process of neurotransmission?
|
3. The presynaptic nerve is stimulated causing the synaptic vesicles to fuse with the synaptic membrane and release the NT by exocytosis (calcium).
4. The NT diffuses across the synaptic cleft and may bind to postsynaptic receptors. |
|
|
What is step 5 and 6 of the process of neurotransmission?
|
5. Binding results in opening of ion channel or activation of second messengers such as cAMP or inositol phosphate.
6. This action causes DEPOLARIZATION. |
|
|
What is step 7 of the process of neurotransmission?
|
7. NT molecules which fail to bind to postsynaptic receptors are destroyed by degradative enzymes, are taken up into the presyn. neuron to be recycled or diffuse away from the synaptic cleft.
|
|
|
Summarize the process of neurotransmission.
|
1. NT synthesized.
2. Stored in vesicles in presyn. termin. 3. Stimulation causes release of the NT by exocytosis. 4. NT diffuses across synaptic cleft. 5. Binding = ion channel opening or activation of 2nd messenger. 6. Depolarization results. 7. Nonbinding results in presynaptic uptake to recycle, destruction of NT by degradative enzymes, or diffusion away from cleft. |
|
|
What are the 3 ways of NE termination? What types of drugs specifically inhibit some of the ways?
|
1. Reuptake-postg. nerve endings (70%). Inhibited by TCA's.
2. Diffusion from receptor sites (MAOI's). 3. Catechol-O-Methyltransferase (COMT) (Inhibited by MAOI's) |
|
|
Postganglionic also means ____.
|
Presynaptic (confusing, ain't it?).
|
|
|
Where is MAO found?
|
Large amounts in the mitochondria of sympathetic neurons as well as liver and intestine.
|
|
|
Where is COMT found?
|
In close proximity to MAO, outside the sympathetic neuron and in large amounts in liver and kidney.
|
|
|
The major metabolite of catecholamine termination?
|
3,4 dihydroxymandelic acid => vanillymandelic acid (VMA)
|
|
|
Where is VMA excreted?
|
Urine.
|
|
|
VMA is used to diagnose ____. Why?
|
Pheochromocytoma. "If too many circulating catacholamines present, there will also be too much metabolite, indicating of pheochromocytoma”
|
|
|
Describe the metabolism of NE and E.
|
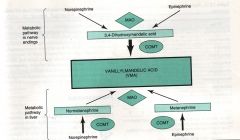
|
|
|
Describe the metabolic pathway of NE and E in nerve endings.
|
NE and E metabolized by MAO to 3,4 dihydroxymandelic acid which is further metabolized by COMT to VMA.
|
|
|
Describe the metabolic pathway of NE and E in the liver.
|
NE and E metabolized by COMT to normetanephrine and metanephrine, then further metabolized by MAO to VMA.
|
|
|
What subtype receptor is a1? What does it activate? What are the effects of activation?
|
Subtype Gq-protein receptor. Activates phospholipase C, 2nd messenger isositol 1,4,5 triphosphate, diacyglycerol. Phospholation of ca+ ion channels affects end organs.
|
|
|
What type of receptor is a2? What are the effects of activation?
|
Subtype Gi-protein receptor. Decreases adenyl cyclase, decreases cAMP, blocks the release of presynaptic neurons, decreases sympathetic outflow.
|
|
|
What types of receptors are B1,B2 and B3? What are the effects of activation?
|
Subtype Gs-protein receptor. Activates 2nd messenger, cAMP in the cells. Cardiac have more B1 than B2. B3 located in adipose tissue.
|
|
|
Where are A1 receptors located?
|
Postsynaptic; smooth muscle, eye, lung, uterus, gut, GU.
|
|
|
What are the CV effects of A1 activation?
|
Vasocx, increased SVR, left ventricular afterload and ABP.
|
|
|
What are the immediate effects of A1 activation at the cellular level?
|
Increases intracellular ca+ ion concentration which leads to muscle contraction.
|
|
|
Where are a2 receptors located?
|
Presynaptic nerve terminals.
|
|
|
What are the effects of a2 activation on a cellular level?
|
Inhibits adenylate cyclase activity which 1. decreases the entry of ca+ ions into the storage vesicles containing NE, 2. thus creating a negative feedback loop that inhibits further release of NE from the neuron.
|
|
|
What is the effect of activation of a2 receptors in the CNS?
|
Sedation, reduction of sympathetic outflow. Leads to vasodilation and decreased BP.
|
|
|
Where are b1 receptors located?
|
Postsynaptic membranes in the heart.
|
|
|
On the cellular level, what effect does activation of B1 receptors have?
|
Activates adenylate cyclase which converts to adenosine triphosphate to cyclic adenosine monophosphate and initiates a kinase phosphorlation cascade.
|
|
|
B1 results in positive ___, ___ and ___.
|
Chronotropy, dromotropy and inotropy.
|
|
|
Where are B2 receptors?
|
Postsynaptic adrenoreceptors. In smooth muscle and gland cells.
|
|
|
Cellular effects of B2 activation?
|
Activates adenylate cyclase, activates Na/K pump.
|
|
|
What importance does B2 activation have in terms of electrolyte imbalance?
|
This receptor activates the Na/K pump, which pushes K intracellularly, possibly resulting in hypokalemia and dysrhythmias.
|
|
|
What are the systemic effects of a1 agonism? (Eyes, salivary glands, heart, lungs, pancreas, upper GI, liver, abdominal blood vessels, bladder).
|
Mydriasis, increased salivary secretion, bronchocx, decreased insulin secretion, upper GI sphincter cx, glycogenolysis, abdominal blood vessel cx, bladder sphincter cx.
|
|
|
What are the sympathetic systemic effects of B1 and B2 agonism? (Eyes, salivary glands, heart, lungs, pancreas, upper GI, liver, abdominal blood vessels, bladder).
|
Ciliary muscle relaxation-far vision (b), positive inotropy, chronotropy and dromotropy (b1), bronchodilation (b2), increased insulin secretion (b2), decreased GI tone and motility (b2), gluconeogenesis (b2), dilation (b2), bladder detrusor relaxation (b2).
|
|
|
What are direct sympathomimetics? What does catecholamine imply? How are they metabolized?
|
Chemicals that contain a catechol and amine group. They attach to the receptor themselves and are synthesized by the body. Metabolism occurs via MAO and COMT.
|
|
|
What drugs are direct sympathomimetics?
|
Epinephrine, norepinephrine, isoproterenol, dobutamine, dopamine, doxylamine.
|
|
|
Describe the receptor binding properties of epinephrine and potency.
|
A and B agonist. Most potent a-adrenergic receptor agonist. 2-10x more potent than norepinephrine, 100x more potent than isoproterenol.
|
|
|
What is the effect of b1 agonism by epinephrine?
|
Increases CO, MO2 demand by increasing contractility and HR (increases rate of phase IV depolarization).
|
|
|
What is the effect of b2 agonism by epinephrine?
|
Vasodilation that may lower diastolic pressure. Relaxes bronchial smooth muscle.
|
|
|
What is the effect of a1 agonism by epinephrine?
|
Decreases splanchnic and renal blood flow. Increases coronary and cerebral perfusion. Increases systolic pressure.
|
|
|
Be concerned when giving epinephrine to a patient with a ____ history.
|
Cardiac.
|
|
|
What are the clinical uses of epinephrine?
|
1. Addition to L.A. solutions to decrease systemic absorption and prolong the duration of the anesthetic.
2. Tx of life-threatening allergic reactions. 3. During CPR as the single most important drug. 4. Continuous infusion to increase myocardial contractility. |
|
|
What are the doses for epinephrine?
|
1. Shock/allergic reaction: 0.5-1mg IV
2. To improve myocardial contractility: 1mg/250ml D5W is 4mcg/ml. Give at 2-20mcg/min. 3. For bronchospasm: 0.1-0.5ml (SQ/IM) of 1:1000 solution. (Racemic inhalation): 0.5ml of 2.25% solution in 2.5-3.5 nss Q1-4 hours for adults. Q4hrs for peds. |
|
|
Local anesthetics contain epinephrine at what concentrations?
|
1:200,000 (5mcg/ml).
1:100,000 (10mcg/ml). 1:10,000 (0.1mg/ml) 1: 1,000 (1mg/ml) |
|
|
Epinephrine should be used cautiously in patients with ___ ___ ___ disease.
|
Hypertensive ischemic heart disease.
|
|
|
What significant problems may occur with epinephrine administration?
|
Cerebral hemorrhage, MI, dysrhythmias.
|
|
|
What anesthetic agent may potentiate dysrhythmias when administered concurrently with epinephrine?
|
Volatile anesthetics, particularly halothane.
|
|
|
Norepinephrine works on which receptors? Rank from most to least in terms of agonism.
|
a>b1>>b2
|
|
|
Test questions: How are catecholamines made? How are they stored? Where is MAO? Where is COMT stored? Where is it broken down? "I'm certainly going to ask these questions for sure."
|
*
|
|
|
Test questions: Ranking of receptor agonism.
|
*
|
|
|
What is the major effect of norepinephrine?
|
Intense vasocx of arterial and venous vessels.
|
|
|
B1 activation by norepinephrine is responsible for what effect?
|
Increase in cardiac contractility.
|
|
|
Other effects of norepinephrine?
|
Increase in afterload results in reflex bradycardia.
No increase in CO. Decrease in renal blood flow and increase in MO2 requirements. |
|
|
What is the major indication of norepinephrine?
|
Refractory shock to maintain perfusion pressure.
|
|
|
Extravasations may cause ___.
|
Tissue necrosis.
|
|
|
What receptors does isoproterenol agonize?
|
Only B.
|
|
|
Effects of isoproterenol administration?
|
Increase in HR, CO, contractility.
Decrease in PVR, diastolic pressure. Increase MO2 demands. |
|
|
Indications for isoproterenol?
|
Stimulate heart and and bronchodilate.
|
|
|
Describe dobutamine receptor preference.
|
b1>b2=a. It is a relatively selective b1 agonist.
|
|
|
Dobutamine has what effects on resistance and why?
|
No change in resistance because it has a low affinity for a1 and b2.
|
|
|
Dobutamine has what effect on PVR and why?
|
Slight decline in PVR by b2 prevents a significant increase in BP.
|
|
|
Other effects of dobutamine?
|
Decrease in left ventricular filling, increased coronary blood flow. Increases in heart rate are less than with other b-agonists.
|
|
|
What type of pt is dobutamine especially useful in? (Esp. in pts w/___ and ___).
|
Pts with CAD and CHF, especially if they have increased PVR and HR.
|
|
|
What receptors does dopamine work on?
|
Dopinergic, a1 and b1.
|
|
|
Low doses of dopamine has what effect?
|
Constricts arterioles in sites other than the brain & kidneys, thus preserving flow to these vital organs.
|
|
|
High doses of dopamine results in what?
|
Constriction of all vessels.
|
|
|
What is the renal dose of dopamine and the prominent receptor agonized?
|
Dopamine receptor. 2mcg/kg/min.
|
|
|
What dose of dopamine predominantly affects b1? What are the effects of this agonism?
|
2-10mcg/kg/min. Increases myocardial contractility, HR, and CO.
|
|
|
What dose of dopamine predominently affects a1? What are the effects of this agonism?
|
10-20mcg/kg/min. Results in increase in PVR and fall in renal blood flow.
|
|
|
If dopamine is dosed a levels greater than 20mcg/kg/min, what results?
|
Release of NE, causing dopamine to have an indirect effect.
|
|
|
What two properties limit the usefulness of dopamine?
|
Its chromotropic and dysrhythmogenic effects.
|
|
|
What are two major indications for using dopamine? What might it be used with?
|
Shock, renal perfusion. May be given with NTG or nipride.
|
|
|
What is dopexamine? How does it compare to dopamine?
|
Analogue to dopamine with less b1 and alpha effects. It is less arrhythmogenic than dopamine.
|
|
|
What renal effects does dopexamine have in comparison to dopamine and why?
|
It has specific effect on renal perfusion because of fewer beta effects.
|
|
|
What is the dose of dopexamine?
|
Start at 0.5mcg/kg/min to 1mcg/kg/min at intervals of 10-15min to a max of 6mcg/kg/min.
|
|
|
What receptors does fenoldopam agonize?
|
Selective DA1 agonist.
Little or no A, B or DA2 activity. |
|
|
What effects are exerted by fenoldopam?
|
Hypotensive effects characterized by decreased PVR, increased renal blood flow, diuresis, natriuresis.
|
|
|
Fenoldopam is associated with what phenomenon at low doses?
|
Reflex tachycardia.
|
|
|
What are the indications for fenoldopam? Why?
|
Pts undergoing cardiac surgery or AAA repair, because of its antihypertensive and renal sparing properties.
|
|
|
How is fenoldopam dosed?
|
By infusion increased by increments of 0.1mcg/kg/min in 15-20 min intervals until target BP achieved.
|
|
|
Why are direct noncatecholamines named so?
|
They have no amine or catechol group.
|
|
|
Describe the serum t1/2 of direct noncatecholamines compared to catecholamines.
|
Longer, because they are not metabolized by MAO or COMT.
|
|
|
Describe general characteristics of direct noncatecholamines.
|
Most agonize B2.
Most are bronchodilators and uterine relaxers except for phenylephrine and methoxamine (vasoxyl) which are potent a1 agonists. |
|
|
What is phenylephrine? what receptors does it stimulate?
|
Direct acting noncatecholamine. Primarily A1 but may stimulate A2 and B receptors at high doses.
|
|
|
What is the primary effect of phenylephrine? What happens to CO, coronary blood flow, and perfusion pressure?
|
Peripheral vasocx with rise in SVR and ABP. Reflex bradycardia may decrease CO. Coronary blood flow and perfusion pressure increase.
|
|
|
How is phenylephrine dosed? How might it be mixed for infusion?
|
50-100mcg boluses.
100mcg/ml solution for infusions. 10mg/250ml NSS=40mcg/ml |
|
|
When would you give phenylephrine?
|
When there is hypotension with tachycardia d/t reflex bradycardia.
|
|
|
What is terbutaline and ritodrine? What receptor do they agonize? What are their indications?
|
Direct noncatecholamines. Primarily B2. Bronchodilator (terbutaline) and relax uterus in near term parturient.
|
|
|
What are the s/e of terbutaline and ritodrine?
|
Crosses placenta.
*May have dose related tachycardia, increased CO. Increases secretion of renin leading to decreased excretion of NA, K and H2O=>may lead to pulmonary edema. |
|
|
What lab abnormalities may occur with terbutaline and ritodrine?
|
Hypokalemia, hyperglycemia.
|
|
|
What should be done in patients receiving terbutaline and ritodrine?
|
Restrict fluids to less than 2L/day, EKG monitor continuously.
|
|
|
Dose of ritodrine?
|
350mcg/kg/min until contractions are inhibited for at least 12 hours followed by oral dose.
|
|
|
Dose of terbutaline?
|
0.25mg SQ-same as epinephrine but longer lasting.
In children, 0.01mg/kg inhalation and metered dose. |
|
|
How much terbutaline is in 1 vial?
|
1mg-be careful not to give entire vial. Dose is 0.25mg SQ.
|
|
|
How do mixed noncatecholamines work?
|
Displace NE from presynaptic terminals and bind to adrenergic receptors.
|
|
|
Name a mixed noncatecholamine and its effects.
|
Ephedrine. Increases HR, BP, contractility and CO, bronchodilates, stimulates CNS and raises MAC.
|
|
|
Compared to epinephrine, describe ephedrine.
|
Longer acting than epinephrine with similar CV effects.
|
|
|
Indications for ephedrine?
|
*Vasopressor during anesthesia-should be used as a temporizing measure while the cause of hypotension is determined and remedied.
*Preferred for OB. |
|
|
Why is ephedrine preferred for OB?
|
Does not increase uterine blood flow.
|
|
|
Dose of ephedrine? Problems with dosing over time?
|
2.5-10mg bolus-adults
0.1mg/kg bolus-children Tachyphylaxis. |
|
|
When is it ideal to use ephedrine over phenylephrine?
|
Hypotension with bradycardia.
|
|
|
Name an indirect noncatecholamine. Describe its effects.
|
*Amphetamine. Causes vasocx d/t release of NE.
*CNS: wakefulness, elation, decreased appetite. |
|
|
What may occur with chronic administration of amphetamine?
|
Depletes CNS stores of catecholamines and may decrease anesthetic requirements.
|
|
|
Describe the differences between direct, indirect and mixed sympathomimetics.
|

|
|
|
What is an A2 agonist also known as?
|
Sympatholytic-sympathetic outflow is reduced.
|
|
|
What is the prototype A2 agonist? How does it work? What is it indicated for?
|
Methyldopa. Relies on metabolites to be effective. Still recommended for tx of HTN in pregnancy.
|
|
|
Name another A2 agonist and its cardiac and CNS effects.
|
Clonidine-antihypertensive. Negative inotrope. Decreases SVR and has sedative properties.
|
|
|
How is clonidine dosed?
|
Oral: 3-5mcg/kg
IM: 2mcg/kg IV: 1-3mcg/kg TD: 0.1-0.3mg/24hours Intrathecal: 75-150mcg Epidural: 1-2mcg/kg |
|
|
How does clonidine interact with anesthetics and blocks?
|
May decrease anesthetic and analgesic requirements.
Prolongs blocks. |
|
|
Other indications for clonidine?
|
Decreases post-op shivering.
Tx of chronic pain syndromes. |
|
|
Common s/e of clonidine?
|
Bradycardia, hypotension, sedation, dry mouth, respiratory depression.
|
|
|
What is precedex? When can it be used?
|
Relatively selective A2 adrenoreceptor agonist. May be administered via infusion before intubation, during intubation and after extubation, but not longer than 24 hours.
|
|
|
Precedex agonizes which receptors on the neuron? What is the affinity for A2 over A1?
|
Pre and post-synaptic A2 adrenoreceptors. 1620:1.
|
|
|
List 6 common effects of precedex.
|
1. Decreased NE levels.
2. Decreased brain noradrenergic activity 3. Sedation 4. Inhibition of sympathetic activity 5. Decreased BP 6. Decreased narcotic requirements. |
|
|
Distribution half-life of precedex? Elimination half-life?
|
6 min.
2 hours. |
|
|
The elimination of precedex follows ___ kinetics.
|
Linear.
|
|
|
What is the dose of precedex?
|
0.2-0.7mcg/kg/hr.
|
|
|
What is the ss VD of precedex? Protein binding?
|
173L. 94%.
|
|
|
Adverse effects of precedex?
|
Hypotension, hypertension during loading infusion, nausea, bradycardia, anemia.
|
|
|
List drug interactions that may occur with precedex.
|
Coadministration of anesthetics, sedatives, hypnotics, and opioids is likely to lead to an enhancement of effects.
|
|
|
Caution should be exercised when administering Precedex to patients with ___ and/or ___.
|
Severe heart block, severe ventricular dysfunction.
|
|
|
These cardiac anomalies with precedex have been noted in what populations and in what circumstances?
|
Clinically significant episodes of bradycardia and sinus arrest have been associated with Precedex administration in young, healthy volunteers with high vagal tone or with different routes of administration including rapid intravenous or bolus administration
|
|
|
This type of drug is selective and exerts competitive inhibitory action on an enzyme of PDE (PDE III).
|
Noncatecholamine nonglycoside cardiac inotropic agent.
|
|
|
How do noncatecholamine nonglycoside cardiac inotropic agents affect cAMP and cGMP?
|
*Decrease the hydrolysis of cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP).
*Increase intracellular cAMP and cGMP in the myocardium and vascular smooth muscle |
|
|
What is the function of cAMP?
|
cAMP stimulates protein kinases that phospholate substances responsible for inward movement of calcium ions.
|
|
|
PDE III -enhance ___ effects. Why?
|
Catecholamine. Because beta stimulation also increases CAMP.
|
|
|
PDE III –act ___of beta receptors.
|
Independently.
|
|
|
PDE III potentially ___ though this is not a predominate effect.
|
Bronchodilates.
|
|
|
PDE III's have positive ___ effects. They increase ___ for contraction but also increase removal of ___ from the ___.
|
Inotropic; calcium; calcium; myoplasm.
|
|
|
What are the cardiovascular actions of cAMP-dependent PDE III inhibitors (regarding systemic circulation and cardiopulmonary effects)?
|
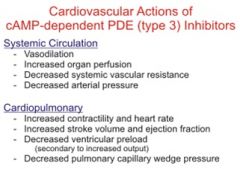
|
|
|
How does cAMP work?
|
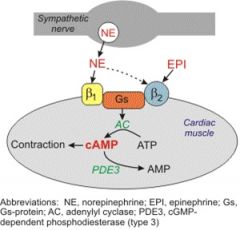
|
|
|
How does cGMP work?
|
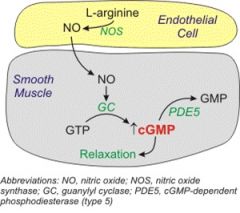
|
|
|
Describe amrinone and its effects.
|
*Bipyridine derivative-PDE III
*Dose dependent positive inotropy-increases CO *Vasodilator-decrease left ventricular end-diastolic pressure *Increase Heart rate (baro reflex) |
|
|
What is the t1/2 and onset of amrinone? How is it eliminated?
|
T1/2=6 hours. Onset 5 minutes.
Excreted unchanged in urine. |
|
|
How is amrinone dosed?
|
IV: 0.5- 1.5 mg/kg or 2-10ug/kg/min
Max daily dose: 10mg/kg |
|
|
Describe milrinone and its effects.
|
Mipyridine derivative: + ionotrope.
Has vasodilating effects. Minimal effects on heart rate and myocardial O2 consumption |
|
|
How is milrinone dosed?
|
IV: 50ug/ kg, 0.5ug/kg/min
|
|
|
What is the t1/2 for milrinone? How is it eliminated?
|
½ time: 2.7 hours.
80% excreted unchanged by the kidneys. |
|
|
What is the major indication for milrinone?
|
Management of LV dysfunction off bypass.
|
|
|
Describe theophylline. What type of drug is it? Indications? Metabolism? S/E? Dose?
|
*Nonselective phosphodiesterase inhibitor.
*Used for bronchodilation. *Metabolized by the liver. *Has narrow therapeutic margin. *S/E: Dysrthymias, crosses the placenta, relaxes GI sphincter. *Loading dose 5mg/kg, 0.5-1.0mg/kg/hr |
|
|
Describe drug type, indications, effects, DOA and dosing of calcium.
|
*Nonselective phosphodiesterase inhibitor.
*+ ionotrope. * Increases SV, decreases LV end diastolic pressure. *Last 10-20 minutes. *IV 5-10mg/kg |
|
|
What is glucagon? What are its indications, doses and side effects?
|
*Polypeptide hormone produced by the alpha cells of the pancreas.
*Increases myocardial contractility and heart rate in the presence of beta blockade., *Dose: IV 1-5 mg, 20mg/hr *SE: NV, hypokalemia, hypertension in the presence of pheochromocytoma. |
|
|
Explain how indirect, direct and mixed sympathomimetics work.
|
1. Direct agents mimic NE as agonists at adrenergic receptors without interacting with the presynaptic neuron. The chemical structure of the drug determines its subclass specificity.
2. Indirect sympathomimetics force epinephrine release from presynaptic terminal, enhancing the actions of endogenous NE. These do not bind to adrenergic receptors. 3. Mixed sympathomimetics induce the release of NE and also bind to adrenergic receptors. Sympathetic neurotransmission is also enhanced by inhibition of the degradative enzyme, MAO. |

