![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
31 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
ClO3 |
Chlorate 1- |

|
|
|
ClO2 |
chlorite 1- |

|
|
|
C2O4 |
oxalate 2- |
|
|
|
ClO4 |
perchclorate 1- |

|
|
|
NO2 |
nitrite 1- |
|
|
|
PO3 |
phosphite 3- |

|
|
|
H3O |
hydronium 1+ |
|
|
|
O2 |
peroxide 2- |

|
|
|
HS |
hydrogen sulfide 1- |
|
|
|
CN |
cyanide 1- |
|
|
|
OH |
hydroxide 1- |
|
|
|
MnO4 |
permanganate 1- |
|
|
|
HPO4 |
hydrogen phosphate 2- |
what is the charge of phosphate? |
|
|
ClO |
hypochlorite 1- |

|
|
|
SO3 |
sulfite 2- |

|
|
|
CrO4 |
chromate 2- |
|
|
|
Cr2O7 |
dichromate 2- |
|
|
|
Hg2 |
mercurous 2+ |
|
|
|
NH4 |
ammonium 1+ |
|
|
|
SCN |
thiocyanate 1- |
|
|
|
IO3 |
iodate 1- |

|
|
|
S2O3 |
thiosulfate 2- |
|
|
|
SO4 |
sulfate 2- |

|
|
|
CO3 |
carbonate 2- |
|
|
|
NO3 |
nitrate 1- |
|
|
|
PO4 |

phosphate 3- |
|
|
|
HCO3 |
hydrogen carbonate 1- |
|
|
|
H2PO4 |
dihydrogen phosphate 1- |
|
|
|
HSO4 |
hydrogen sulfate, 1- |
|
|
|
C2H3O2 |
acetate 1- |
|
|
|
name all of the naturally occurring diatomics |
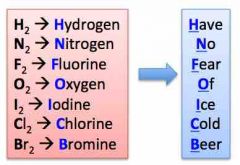
Back (Definition) |
|

