![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
85 Cards in this Set
- Front
- Back
|
Characteristics of Mitochondria |
Double membrane bound organelle Mitochondrial DNA is present Contains large number of integral proteins Postulated to have arisen from endosymbiosis site of ATP production found in nearly all eukaryotic cells contains two membranes: cristae and matrix |
|
|
Endosymbiosis |
Evolutionary theory which entertains the concept that eukaryotes evolved from prokaryotes by consuming prokaryotes and they essentially live inside one another |
|
|
Microtubules |
made of alpha and beta tubulin monomers thickest and form hollow tubes involved in support, organelle transport, cell division and movement Grows from centrosomes constituent of cilia and flagella |
|
|
Organelles for energy production |
produces usable chemical energy for the cell from other sources energy stored in chemical bonds include mitochondria and chloroplast |
|
|
Peroxisomes |
Breakdown of peptide bonds in metabolism |
|
|
peroxisomes |
digest lipids |
|
|
lysosomes |
hydrolyze peptides in the cytosol Digest worn out organelles, food particles engulfed bacteria/virus |
|
|
proteosomes |
hydrolyze extracellular peptides Degrades unneeded or misfolded proteins through proteolysis, which is hydrolysis, specifically for proteins. |
|
|
ATP is a result of |
respiration occuring in the mitochondria |
|
|
Mitochondria membranes |
inner: convoluted and contains some respiratory enzymes internal space: contains mtDNA, respiratory enzymes, and ribosomes. high surface to volume ratio |
|
|
mitochondrial matrix |
found in the inner membrane interior or mitochondria created by inner membrane |
|
|
Cristae |
found in the inner membranecreates the folds found within the mitochondria contains proteins and molecules used for making chemical energy. |
|
|
Endoplasmic Reticulum |
network of sacs and tubules (cisternae) continuous with the outer nuclear membrane Interior: (cisternal space) continuous with space between nuclear membranes smooth and rough |
|
|
Smooth ER |
lipid synthesis, carbohydrate metabolism, calcium storage and detoxification |
|
|
rough |
ribosomes on cytoplasmic face site of protein synthesis and modification (glycoproteins) vesicles bud and go to Golgi |
|
|
Liver |
Organ with a function similar to the smooth ER. Detoxifies alcohols and medicines |
|
|
cytosol |
fluid within the cell membrane. |
|
|
Constituents Endomembrane system |
Nucleus, ER, Golgi, lysosomes, vesicles |
|
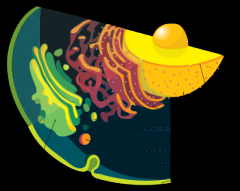
|
Endomembrane system with labels |
|
|
Golgi apparatus |
destination of most ER vesicles modify ER products, store temporarily before sending elsewhere synthesizes some polysaccharides |
|
|
Golgi apparatus constituents |
Stacked cisternae cis: recieves ER vesicles trans: finished products from Golgi processing vesicles |
|
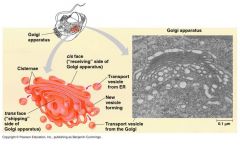
|
Golgi apparatus and constituents |
|
|
Functions of endoplasmic reticulum |
Folds proteins molecules in cisternae transports synthesized proteins in vesicles to the Golgi apparatus |
|
|
Vesicles |
small transport structure made of a phospholipid bilayer |
|
|
Function of ER and Golgi apparatus |
modify plasma membrane lipids and proteins *maybe* |
|
|
Lysosomes |
acidic compartment (pH approximately 5) used to digest macromolecules controls cells death (cell lysis) |
|
|
what forms lysosomes
|
trans Golgi phagocytosis recycled cellular material |
|
|
Proteasomes |
Large protein complex Degrades proteins into polypeptides which get recycled to amino acids |
|
|
degredation of proteins control function |
Mitosis Apoptosis Cell signaling and communication Gene expression Immunity |
|
|
Vacuoles |
important in storage and homeostasis |
|
|
Central vacuole in plants |
contractile and vacuole |
|
|
Peroxisomes |
transfer H to O2 (2H+O2-->H2O2) important in digesting some molecules (lipids) does not form by budding from Golgi often cooperate with energy organelles |
|
|
Chloroplast function |
site of photosynthesis (conversion of light energy into chemical) important for carb production |
|
|
Chloroplast structure |
double membrane with internal membranous structure (thylakoid) thylakoids found in stroma |
|
|
stroma |
thylakoids found here; stroma also contains chloroplast DNA, Riboslmes and enzymes for protein synthesis. connective tissue and structural framework of chloroplast |
|
|
thylakoid |
site of light dependent reactions in photosynthesis |
|
|
granum |
stacks of thylacoids |
|
|
Endosymbiotic theory |
big cell ate little cell to give rise to some organelles proteins within made by ribosomes within organelle or by free ribosomes grow and reproduce on own encased in double membrane maintain their own DNA same size as prokaryote |
|
|
Keratin |
Supercoiled fibrous proteins |
|
|
Free Energy |
total energy + entropy deltaG= deltaH-T*deltaS |
|
|
As free energy increases stability ____ |
decreases |
|
|
A spontaneous reaction occurs under what conditions |
deltaG < 0 (releases energy) Enthalpy decreases while entropy increases |
|
|
Catabolic metabolism |
Releases energy used to drive chemical reactions. Breakdown of complex organic molecules into simpler ones Free energy: G< 0 releases energy Energy released supports anabolic reactions |
|
|
Anabolic metabolism |
Simpler substances combine to form more complex molecules Requires input energy Non spontaneous G>0 |
|
|
Equilibrium |
G=0 |
|
|
Chemical reactions in cells are usually |
in equilibrium |
|
|
Chemical reactions in equilibrium are not favorable to living organisms because |
No work can be done in equilibrium reactions |
|
|
Exergonic |
Energy is released= catabolism |
|
|
Endergonic |
Energy is absorbed = anabolism |
|
|
Hydolysis of Sugar |
C6H12O6-> 6H2O+6CO2 deltaG= -686 kcal/mol exergonic |
|
|
Synthesis of sugar |
6H2O+6CO2+light-> C6H12O6+ 6CO2 endergonic 686 kcal/mol |
|
|
When deltaG is negative the reaction is considered |
catabolic, exergonic |
|
|
Anabolic |
building up/synthesis endergonic reactions, anabolic, requires energy |
|
|
more covalent bonds = |
more energy |
|
|
Closed system |
equilibrium |
|
|
Open system |
interacts with the environment energy can be transferred |
|
|
ATP |
Adenosine triphosphate. Energy currency of the cell. Adenosine, nucleotide, building, building blocks for RNA Allows work, transport |
|
|
Phosphoanhydride bonds |
Produces a lot of energy, exergonic. 3 found in the bonds of ATP, allows for quick energy transfer |
|
|
Reaction of ATP |
ATP-->ADP+Pi |
|
|
Energy of hydrolysis of ATP |
G: -7.3 kcal/mol |
|
|
Negative deltaG allows for |
Allows for a chain of chemical reactions |
|
|
Reaction coupling |
Coupling endergonic and exergonic reactions to power each other. |
|
|
Phosphate transfer in ATP |
leads to a reactive intermediate |
|
|
Free energy must be negative |
for a reaction to proceed |
|
|
Gamma |
Bond farthest away from the ribose sugar in ATP. Extremely high energy and unstable |
|
|
Phosphodiester bond |
2 bonds broken in ATP. ASK DR. BARTON FOR CLARIFICATION |
|
|
Cyclic nature of ATP |
cellular respiration catabolic releases energy and breaks up ATP to ADP +Pi which is then recombined into ATP during anabolism |
|
|
Spontaneity |
Cells resist spontaneously occuring reactions by requireing activation energy |
|
|
Transition state |
Point in a chemical reaction in which activation energy requirement is met, allowing it to take place. |
|
|
Enzymes |
Lower activation energy required for a chemical reaction to take place |
|
|
Enzymatic properties |
Mostly proteins that decreases activation energy IT DOES NOT CHANGE FREE ENERGY, only lowers activation energy |
|
|
Active site |
where a substrate reaction occurs. |
|
|
Enzymes bond at the ___ to cause ___ |
active site to cause an induced fit. Like keys to a lock. Forms covalent bonds. |
|
|
Enzymes are mostly |
Proteins, but some nucleic acids |
|
|
Enzyme action in hydrolysis of sucrose |
1) enzyme available w/ empty active site 2) substrate binds to enzyme forming the enzyme/substrate complex 3) substrate converted to products and is released |
|
|
Enzyme also ___ reactions |
orients. |
|
|
Amino acids |
can function both as an acid or as a base. |
|
|
Microenvironment |
facilitates rxn, right where rxn is occuring |
|
|
Rate of enzymatic activity is dependent on the |
availability of the active size. |
|
|
Denaturation |
Occurs when anything influence the protein structure. pH, temperature, etc. |
|
|
cofactors |
non-protein helpers, cannot be transient of permanent. Usually metals, minerals |
|
|
coenzyme |
organic cofactors they are necessary rxn will not occur otherwise |
|
|
Factors effecting enzyme activity |
inhibitors |
|
|
competitive inhibitor |
competes with the enzyme for active site, which has a higher affinity that prevents the substrate from binding |
|
|
noncompetitive |
bonds to another region to disrupt activity |

