![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
34 Cards in this Set
- Front
- Back
|
How do particles move in solids? |
The particles stay in one place and vibrate back and forth |
|
|
How do liquid particles move? |
The particles move past each other and move around but stay close together |
|
|
How do gas particles move? |
The particles bounce around the space they are in and spread out to fill the container |
|
|
How are solid particles spaced? |
The particles are close together |
|
|
How are liquid particles spaced? |
The particles are close together but further apart than in solids |
|
|
How are gas particles spaced? |
The particles are far apart |
|
|
Do solids have a definite or indefinite shape? |
Definite |
|
|
Do liquids have a definite or indefinite shape? |
Indefinite |
|
|
Do gasses have a definite or indefinite shape? |
Indefinite |
|
|
Do solids have a definite or indefinite volume? |
Definite |
|
|
Do liquids have a definite or indefinite volume? |
Definite |
|
|
Do gasses have a definite or indefinite volume? |
Indefinite |
|
|
Which states are fluids? |
Liquids and gasses |
|
|
What is a fluid? |
Changes shape and flows |
|
|
What is plasma? |
Like a gas but particles have so much energy they are falling apart and making light |
|
|
What is viscosity? |
How thick it is |
|
|
What is temperature? |
The average kinetic energy of particles in peice of matter |
|
|
What's part 1 of the kinetic theory? |
All substances are made of particles that are constantly moving |
|
|
What is part 2 of the kinetic theory? |
When you raise the temperature of a substance the particles speed up |
|
|
What is part 3 of the kinetic theory? |
Particles are constantly colliding with each other and their container |
|
|
What happens to pressure if you increase the volume of a gas? |
Pressure decreases |
|
|
What happens to pressure if you increase the temperature of the gas? |
Pressure increases |
|
|
How can you raise the boiling point of a liquid, like water? |
Increase the pressure |
|
|
What is the most common state of matter in the universe? |
Plasma |
|
|
Define absolute zero |
When particles have 0 kinetic energy |
|
|
What is the name of the temperature scale that starts at absolute zero? |
Kelvin |
|
|
How how many degrees higher than absolute zero is 0° Celsius |
273° C |
|
|
How is pressure related to the movement of gas particles? |
Pressure is caused by collisions of gas particles with each other and their container |
|
|
What is the difference between temperature and heat? |
temperature is an average of the kinetic energy of particles, he is an absolute value so it changes if you have a small / large amount |
|
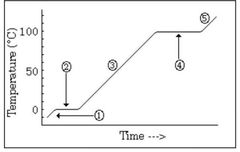
The water is in a ________ state and the temperature is ________ |
Solid, going up |
|
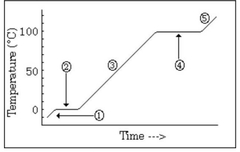
The water is in a ________ state and the temperature is ________ |
Melting, constant |
|
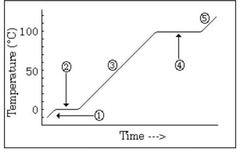
The water is in a ________ state and the temperature is ________ |
Liquid, going up |
|
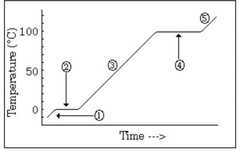
The water is in a ________ state and the temperature is ________ |
Boiling, constant |
|
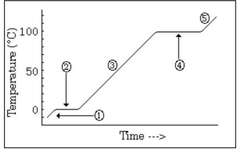
The water is in a ________ state and the temperature is ________ |
Liquid, going up |

