![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
18 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Solid
|
A state of matter that has a definite shape and a definite volume.
|
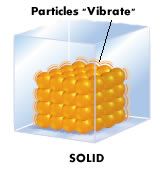
|
|
|
Crystalline Solid
|
Solid that is made up of crystals in which particles are arranged in a regular, repeating pattern.
|
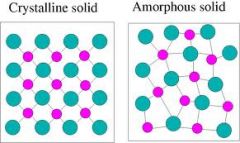
|
|
|
Amorphous Solid
|
A solid made up of particles that are not arranged in a regular pattern.
|
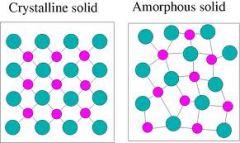
|
|
|
Liquid
|
A state of matter that has no definite shape but has a definite volume.
|
|
|
|
Fluid
|
A substance that can easily flow.
|
|
|
|
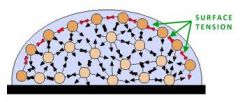
Surface tension
|
The result of an inward pull among the molecules of a liquid that brings the molecules on the surface closer together.
|
|
|
|
Viscosity
|
A liquid's resistance to flowing.
|

|
|
|
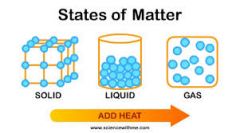
Gas
|
A state of matter that has no definite shape and no definite volume.
|
|
|
|
High viscosity
|
a fluid that doesn't pour easily (like honey)
|

|
|
|
low viscosity
|
a fluid that has a high rate of flow (pours quickly). Ex: motor oil
|
|
|
|
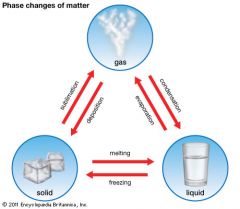
Changes of State.
|
Physical changes of matter to a different state (solid, liquid, gas).
|
|
|
|
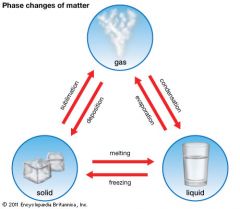
Freezing
|
Process in which a liquid changes into a solid.
|
|
|
|
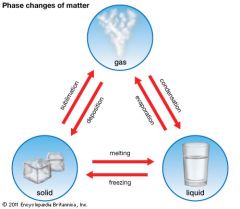
Melting
|
The process in which a solid changes into a liquid.
|
|
|
|
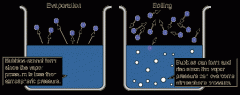
Vaporization
|
Process in which a liquid boils and changes into a gas. You would see bubbles of water vapor escaping.
|
|
|
|
Evaporation
|
Process where a liquid changes into a gas without boiling. You would NOT see bubbles of water vapor
|
|
|
|
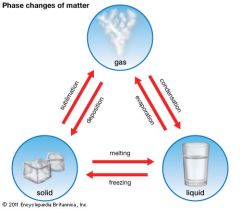
Condensation
|
Process in which a gas changes into a liquid.
|
|
|
|
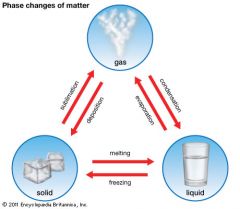
Sublimation
|
Process in which a solid changes into a gas.
|
|
|
|
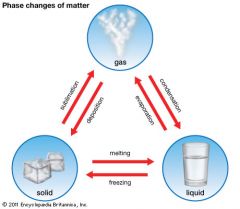
Deposition
|
Process where a gas changes directly into a solid.
|
|

