![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
15 Cards in this Set
- Front
- Back
|
Difference between Crystalline solid and amorphous solid |
1] Regularity and periodicity in arrangement of particles a] Random arrangement of particles 2] Sharp melting point b] don't have a Sharp melting point 3] Shows anisotropic nature (physical properties shows different in different directions) c] Shows Isotropic nature 4] eg... Ice, NaCl, Au, Na, Diamond d] eg... Rubber, Plastic, Glass |
|
|
What is isomorphism ? |
Two or more substances having the same crystalline structure eg... 1. NaF & MgO 2. NaNo3 & CaCO3 |
|
|
What is Polymorphism |
Single substances exist in 2 or more crystalline structure eg... Diamond, Graphite, Fullerene {C60} |
|
|
Define unit cell |
The smallest repeating unit of crystalline solid is called unit cell |
|
|
Write the parameters of unit cell using diagram |
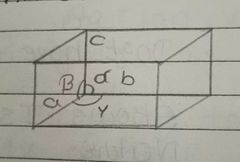
a,b,c = edge length alpha, beta, gyama = angles between two edges |
|
|
Write the type of cubic unit cell |
1] Simple cubic unit cell (SCC) 2] Body centre cubic unit cell (BCC) 3] Face centre cubic unit cell (FCC) |
|
|
In simple cubic unit cell where and how many particles are present |
In SC particles are at its corners and 1/8th part of each sphere occupied in unit cell. hence number of particle in SC is N = 1/8 × 8 = 1 |
|
|
In body centre cubic unit cell where and how many particles are present |
In BCC one particle is at the corner of the cube in addition to particle at eight corners, hence number of particle in BCC N = 1+1 = 2 = 1/8 × 8+1 |
|
|
In FCC where and how many particles are present |
In FCC one particle at 6 faces occupies 1/2 part is in addition to 8 corners N = 1/8×8+1/2×6 = 1+3 = 4 |
|
|
Define bravais lattice |
Mathematical analysis prove that only 14 different ways to arrange particles in crystal structure called Bravais Lattice |
|
|
Write the types of crystal system |
1] Cubic - 3 2] Orthorhombic - 4 3] Tetragonal - 2 4] Monoclinic - 2 5] Rhombohedral - 1 6] Triclinic - 1 7] Hexagonal - 1 |
|
|
Write the formula who gives relation between molar mass, density and edge length |
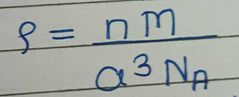
n = number of particles in unit cell (F-4, S-1, B-2) M = molar mass a = edge length NA = Avagadro's number ( 6.022 × 10^22 ) Last symbol= density |
|
|
Define packing efficiency |
The percentage of total space occupied by sphere is called packing efficiency. |
|
|
Write the formula packing efficiency |
Packing Efficiency = total volume of particle in unit cell × 100 / total volume of unit cell |
|
|
No. of particles in x g = Number of unit cells in the V volume of metal = Number of unit cells in x g = |
No. of particles in x g = xn/density ×a^3Number of unit cells in the V volume of metal = V/a^3Number of unit cells in x g = x/density × a^3 |

