![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
308 Cards in this Set
- Front
- Back
- 3rd side (hint)
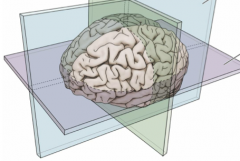
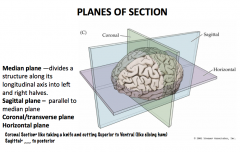
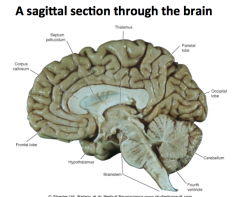
What are the 3 planes? What defines them?
|
|
|
|
|
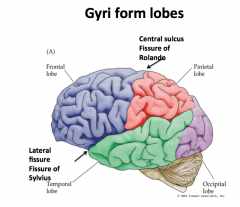
Central Sulcus
|
"Fissure of Rolando"
Separates the frontal lobe from the parietal lobe |
|
|
|
Lateral Fissure
|
"Fissure of Sylvius"
Separates the frontal and parietal lobes from the temporal lobe |
|
|
|
What makes up the brainstem?
|
Midbrain, pons, and medulla
|
|
|
|
Medulla
|
Continuous with the spinal cord and controls heart rate and respiration.
A hit to the back of the head may cause respiratory or cardiac arrest |
|
|
|
Pons
|
A relay center between the cerebrum and cerebellum.
|
|
|
|
Midbrain
|
Connects the cerebral cortex and spinal cord
|
|
|
|
Cerebellum
|
"Little Brain"
Responsible for both fine motor and gross motor control |
|
|
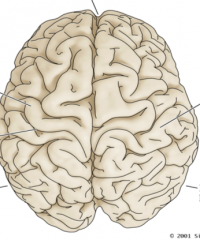
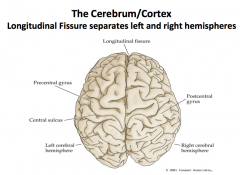
Label.
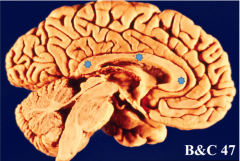
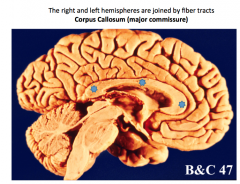
What divides the right and left hemispheres? |
|
|
|
What is this?
|
|
|
|
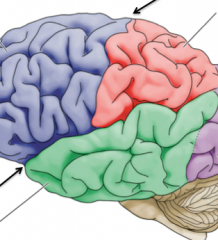
What are Gyri?
|
"folds" in the brain
|
|
|
|
What is the function of the frontal lobe?
|
Anterior Portion- Planning, Reasoning
Posterior Part- Motor Control |
|
|
|
What is the function of the temporal lobe?
|
Dorsal/Posterior- hearing (injury can cause impaired hearing or deafness)
Medial Part- memory processing Anterior Part- higher order visual and auditory processing, semantic processing |
|
|
|
What is the function of the parietal lobe?
|
Primary sensory area- nerve impulses related to pain, temperature, tough and pressure.
Attention, spatial processing. |
|
|
|
What is the function of the occipital lobe?
|
Processing of visual information. Damage here can cause partial or complete blindness.
|
|
|
|
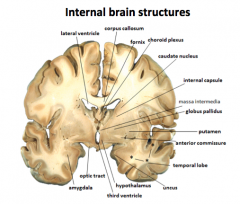
What is the Basal Ganglia?
|
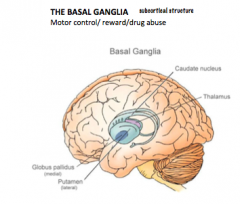
It is involved in motor control and is comprised of the Globus Pallidus and Caudate Putamen.
It is also a circular structure that "hugs" the thalamus. |
|
|
|
Limbic System
|
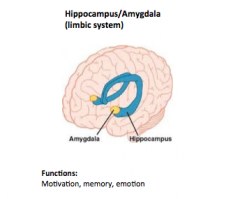
Located sub cortically, forming a C-shaped structure around the thalamus.
|
|
|
|
Ventricular System: Describe the formation and flow route of CSF
|
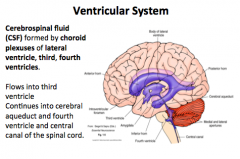
Flow:
Lateral Ventricle → 3rd Ventricle → Cerebral Aqueduct → 4th Ventricle → Central Canal → Spinal Cord |
|
|
|
What is the function of CSF?
How does CSF exit the brain? |
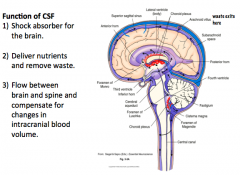
|
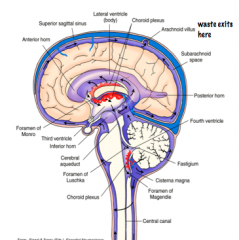
CSF exits the brain from the subarachnoid space, through Arachnoid Villi, into sinuses, eventual ending up in the Venous System
|
|
|
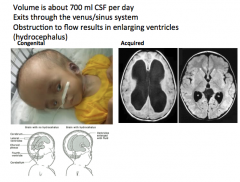
What happens when CSF can't exit the brain?
|
HYDROCEPHALUS
-the enlargement of ventricles Acquired hydrocephalus is more dangerous than congenital hydrocephalus because congenital occurs in children, whose skull bones haven't hardened yet. |
|
|
|
What is a lumbar puncture?
|
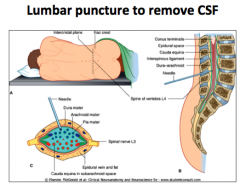
To remove CSF (like if you want to test for meningitis).
Performed around the L4 area, where the Cauda Equina is located. This procedure does not cause damage to the spinal cord because it is like "trying to hit spaghetti" |
|
|
|
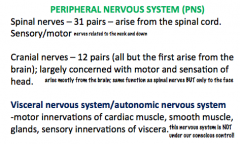
What are the components of the PNS?
|
|
|
|
|
What are the meninges?
|
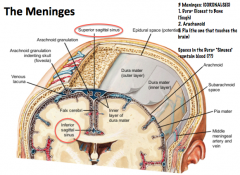
The meninges are a series of membranes that protect the CNS. From outer to inner they are the Dura Mater, Arachnoid, and Pia Mater.
Hemorrhage into the subarachnoid space, where CSF is located, can prevent drainage and cause compression of the brain! |
|
|
|
|
|
|
|
|
|
|
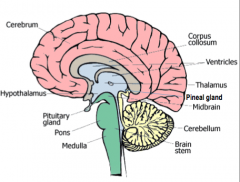
Thalamus
|
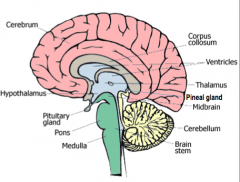
All the senses except smell stop in the thalamus before proceeding into the hemispheres
|
|
|
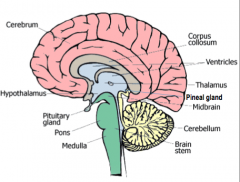
Hypothalamus
|
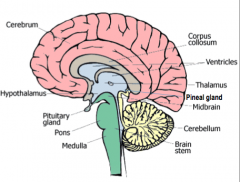
Controls the visceral nervous system (which stimulates contraction of muscle fibers and glandular secretions of internal organs), regulates appetite, thirst, and temperature.
Controls hormonal secretions from the pituitary. |
|
|
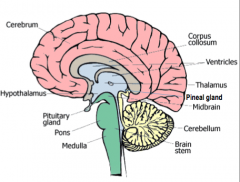
Pineal Gland
|
Biological Clock
|
|
|
|
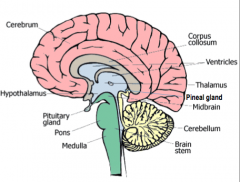
What are the components (4) of the brainstem? What are their functions?
|
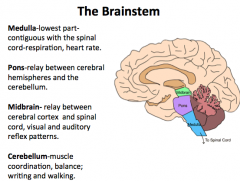
|
|
|
|
What is the spinal cord? What does it do?
|
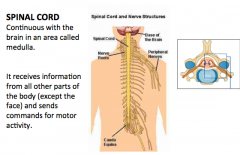
|
|
|
|
What are the spinal cord pathways?
|
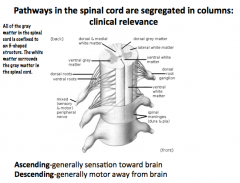
|
|
|
|
What are the 4 major features of Glial Cells?
|
1. *Do NOT directly propagate action potentials!*
2. DO retain the ability to divide 3. Make up the majority of CNS cells 4. Derived from neuroectoderm or mesoderm |
Glia can be distinguished from neurons because Glia DO NOT PROPAGATE ACTION POTENTIALS!
|
|
|
Name the 5 classes of CNS Glia
|
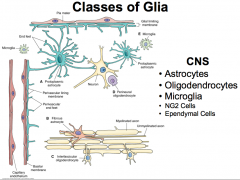
Divided into classes based on their morphology, function, and location within the nervous system (i.e. the PNS or CNS)
Glia make up the majority of CNS cells! And the amount of glia present in an animal is proportionally related to the animal's size! |
|
|
|
Name the 2 classes of PNS Glia
|
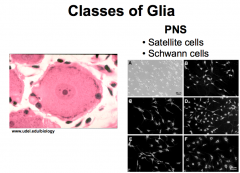
|
|
|
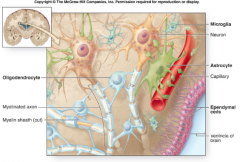
9 Features of Astrocytes (CNS)
(i.e. Shape, Brain Volume, location, derived from, distinguishing characteristics |
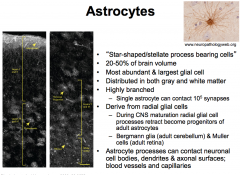
1. Star-shaped/stellate process bearing cells
2. 20-50% of brain volume 3. most abundant and largest glial cells 4. found in both gray and white matter 5. highly branched 6. derived from radial glial cells 7. Astrocyte processes can contact neuronal cell bodies, dendrites, axonal surfaces, and blood vessels 8. regional heterogeneity 9. distinguished from other CNS cells by the presence of glial fibrillary acidic protein (GFAP) |
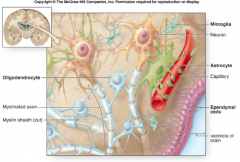
During development of the CNS, radial glial cell processes retract and become progenitors of adult astrocytes.
|
|
|
How can you distinguish Astrocytes from other CNS cells? Name the types of Astrocytes
|
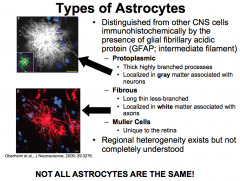
Astrocytes are distinguished from other CNS cells because they make GFAP, which is an intermediate filament.
We know that regional heterogeneity exists because certain types of brain tumors only develop in one region of the brain (i.e. glioblastomas of the temporal lobe). |
|
|
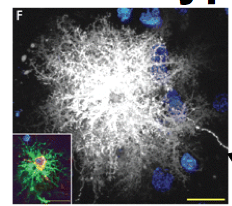
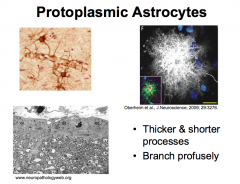
What are Protoplasmic Astrocytes? What are their "end-feet"?
|

Protoplasmic astrocytes are located in Gray Matter. And therefore associated with neurons.
They serve as passages for the transfer of nutrients (predominantly glucose) and oxygen from the blood to neurons. |
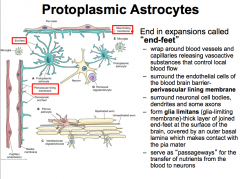
Projections may end in expansions called "end-feet". The end-feet can wrap around blood vessels to form the PERIVASCULAR LINING MEMBRANE.
Astrocytes can release vasoactive substances from the perivascular lining to control local blood flow through. The end-feed can also cover the basal lamina of the pia mater to form the GLIA LIMITANS, which helps to protect the brain. |
|
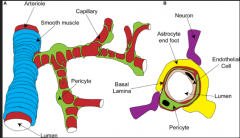
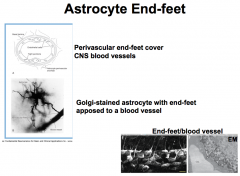
Astrocyte End-Feet
-What are they? -What are their functions? |
|
FUNCTIONS:
1. Wrap around blood vessels and capillaries, and release vasoactive substances, which control local blood flow. -this is important because they can send signals, like when the brain needs more oxygen and thus increased blood flow. 2. They also create the PERIVASCULAR LINING MEMBRANE, which surrounds the endothelial cells of the blood-brain barrier. -this is important for the control of the passage of small molecules because there are spaces between the end-feet (i.e. they don't form tight junctions). 3. Additionally, they surround neuronal cell bodies, dendrites and some axons. 4. They also form the GLIA LIMITANS (aka "glia-limiting membrane). -this membrane is a thick layer of end-feet that are joined at the surface of the brain and covered by an outer basal lamina that makes contact with the pia mater. Thus, they are present in many different areas and have different functions in each place, but in general, the *PROTOPLASMIC ASTROCYTES SERVE AS A PASSAGEWAY FOR THE TRANSFER OF NUTRIENTS FROM THE BLOOD TO NEURONS!* |
|

Protoplasmic Astrocytes: Rodents vs. Humans
|
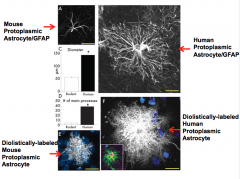
The diameter of the human protoplasmic astrocyte is 2.5 times that of the rodent protoplasmic astrocyte!
The number of processes of the human protoplasmic astrocyte is 10 times that of the rodent protoplasmic astrocyte. Due to this, an impact found in a rodent model may have a larger impact in a human model! |
Differences give us insight into why the capacity of the human brain is so different from that of the rodent brain.
One such difference is the presence of VARICOSE PROJECTION ASTROCYTES in the brains of non-human primates and humans, that are not found in rodents! These projections are tortuous and twisted, and within the webs of inter laminar fibers there are lots of mitochondria, which signifies that a process is occurring that requires a lot of energy! |
|
What are Fibrous Astrocytes?
What is there function? |
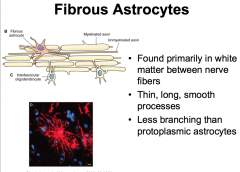
Associated with axons.
|
Respond to injury: reactive astrogliosis. When activated, this type of astrocyte undergoes morphofunctional remodeling. They then proliferate and wall off the damaged area; this process is referred to as Anisomorphic Astrogliosis.
They also communicate with distal neurons to facilitate neuronal remodeling. This process is referred to as Isomorphic Astrogliosis. |
|
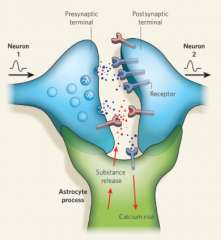
Structural Organization of Astrocytes and Neurons
|
The astrocyte configuration helps them to perform functions like getting rid of NTs by metabolizing them or sucking them up
They can also release their own gliotransmitters such as Glutamate, D-Serine, ATP, and TNFalpha. |
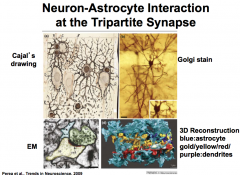
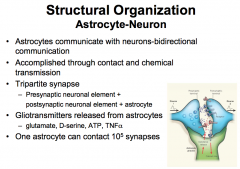
Astrocytes communicate with neurons via bidirectional communication that is accomplished through contact and chemical transmission.
The connection that is created is referred to as a TriPartite Synapse that consists of: *presynaptic neuronal element + postsynaptic neuronal element + astrocyte* |
|
|
List the 7 functions of Astrocytes (CNS)
|
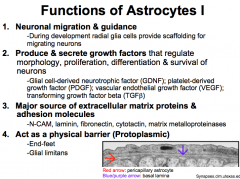
1. Neuronal Migration and Guidance
2. Produce and secrete Growth Factors 3. Major Source of Extracellular Matrix Proteins and Adhesion Molecules 4. Act as a physical barrier (Protoplasmic Astrocytes) 5. Respond to injury: Astrogliosis (Astrocytosis) (Fibrous Astrocytes) 6. Maintain brain homeostases; control blood flow; rapidly remove neurotransmitters & ions; Buffer the extracellular space; Provide energy and substrates for neurotransmission (ATP and Glutamate-Glutamine Cycle) |
|
|
|
What role do Astrocytes play in responding to injury?
|
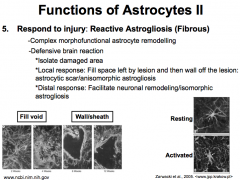
Reactive Astrogliosis is complex morphofunctional astrocyte remodeling, and it is accomplished mostly by the Fibrous Astrocytes.
It occurs via a defensive brain reaction. First, the astrocytes isolate the damaged area. Then they elicit a local response by proliferating to fill in the void left by the wound and then blocking off the lesion (creating a "astrocytic scar"/"an isomorphic astrogliosis"). Lastly, the astrocytes elicit a distal response by releasing things like growth factors to facilitate neuronal remodeling (aka "isomorphic astrogliosis") |
|
|
|
What role do astrocytes play in maintaining brain homeostasis?
|
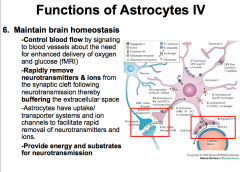
|
|
|
|
What is the Glutamate-Glutamine Cycle in Astrocytes?
|
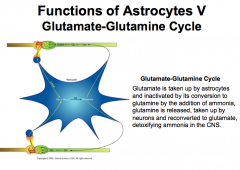
It is important for the brain to be able to detoxify the extra glutamate or it will build up as a neurotoxin.
Just another example of how important astrocytes are! |
|
|
|
What role do astrocytes play in formation and modulation of synapses?
|
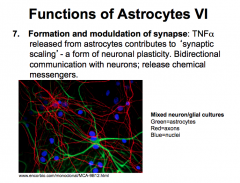
FORMATION OF SYNAPSE
-important in formation bc astrocytes release TNFalpha, which contributes to *Synaptic Scaling*, a form of neuronal plasticity. |
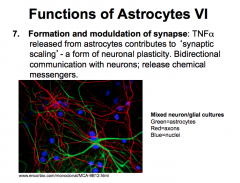
MODULATE SYNAPTIC FUNCTION
-astrocytes participate in bidirectional communication with neurons, release chemical messengers and act as signaling (not action potential producing!!!) cells themselves in order to control message propagation between neurons. |
|
|
What role do astrocytes play in pathogenesis?
|
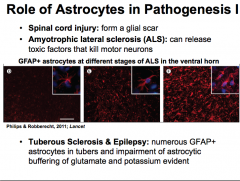
|
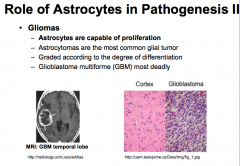
|
|
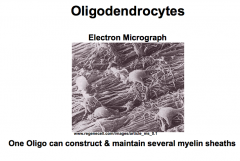
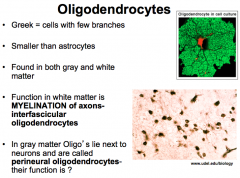
What are Oligodendrocytes? (Features and Functions)
|
|
|
|
|
Oligodendrocytes: Why is Myelination important?
|

|
|
|
|
How is Myelin formed?
|
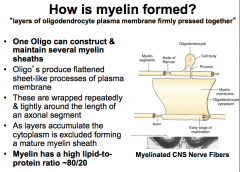
|
|
|
|
What roles do oligodendrocytes play in pathogenesis?
|
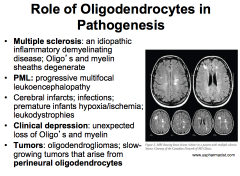
MS- leads to delayed motor function.
|
|
|
|
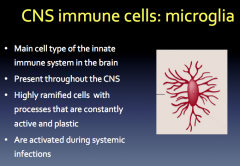
What are Microglia?
|
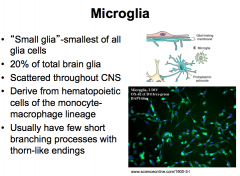
Microglia are very unique because they possess the ability to quickly adapt, which makes them important during development.
|
|
|
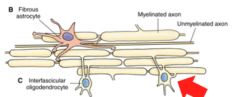
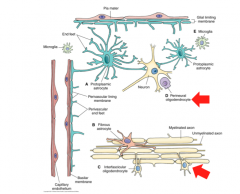
What are Interfasicular Oligodendrocytes? (Location? Function?)
|
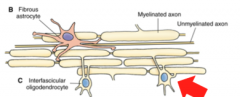
Location- White Matter
Function- Myelination of Axons Note- a single oligodendrocyte can construct and *maintain several myelin sheaths* |
|
|
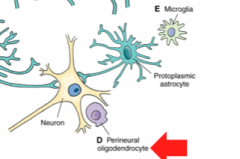
What are Perineural Oligodendrocytes? (Location? Function?
|
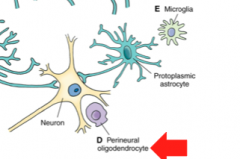
Location- Gray Matter adjacent to neurons
Function- unknown! |
|
|
|
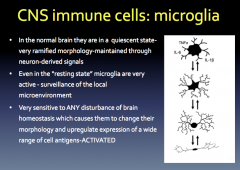
Resting vs. Active Microglia
|
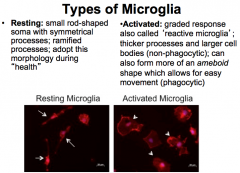
Microglia can become ACTIVATED when they are needed to combed damage! This response is graded and is also known as "Reactive Microglia"
During the non-phagocytic stage, the cells develop thicker processes and larger bodies. Then, during the phagocytic stage, they form more of an ameboid shape, which allows for easier movement to the damaged site. |
|
|
|
Functions (5) of Microglia (CNS)
|
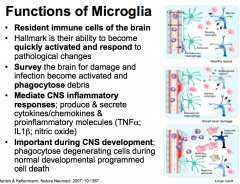
|
|
|
|
What role do Microglia play in pathogenesis?
|
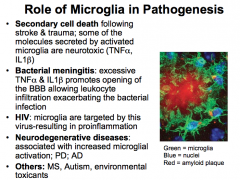
|
|
|
|
What are NG2 Cells (CNS)?
|
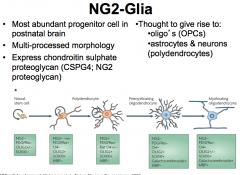
|
|
|
|
What are the 3 types of Ependymal Cells (CNS Glial Cells)?
|
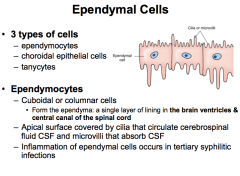
|
|
|
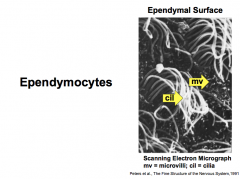
Features/Functions?
|
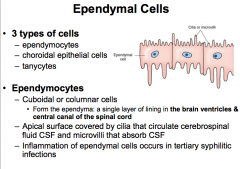
An Ependymal Cell (CNS Glial Cell)
|
|
|
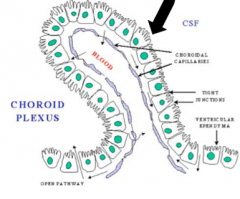
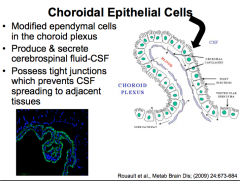
Choroidal Epithelial Cells
|
Ependymal Cells (CNS)
*function to produce and secrete CSF!* |
|
|
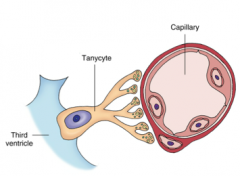
Tanycytes
|
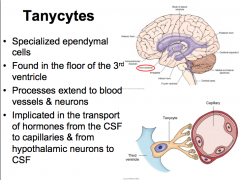
|
|
|
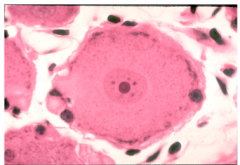
PNS Glia: Satellite Cells
|
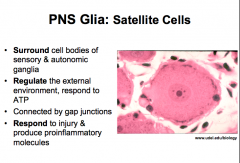
similar functions to astrocytes and microglia!
|
|
|
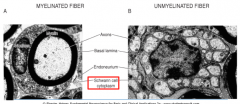
PNS Glia: Schwann Cells
|
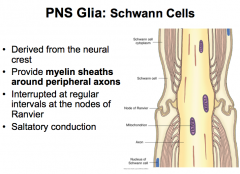
|
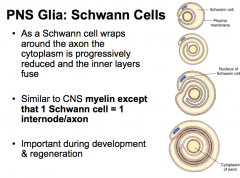
|
|
|
Aside from myelination, what are some other roles of PNS Schwann Cells?
|
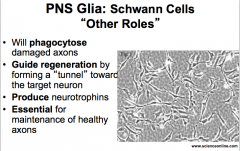
|
|
|
|
What role do Schwann Cells play in pathogenesis?
|
|
|
|
|
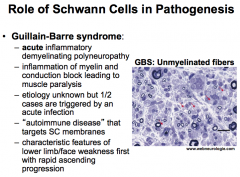
What is Guillain-Barre Syndrome?
|
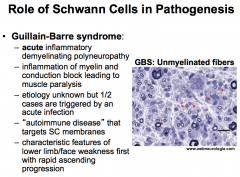
|
|
|
|
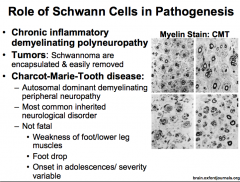
What is Charcot-Marie-Tooth Disease?
|
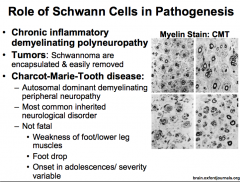
|
|
|
|
What are Gliomas?
|
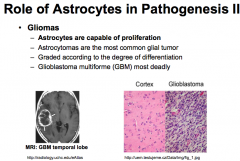
|
|
|
|
What are neurons? What is their function? Characteristics?
|
|
|
|
|
What are the structural components of neurons?
|
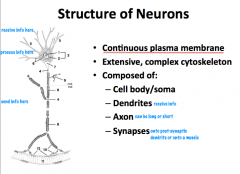
|
|
|
What role does a neuron's cytoskeleton play? What is it composed of?
|
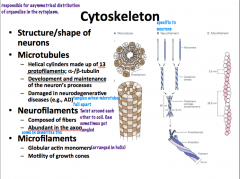
|
|
|
|
What can happen when neurofibrils get tangled?
|
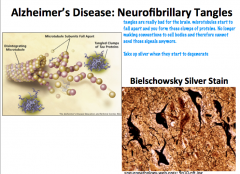
|
|
|
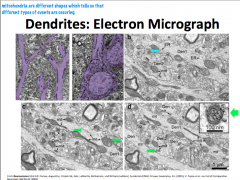
What is the structure and function of dendrites?
|
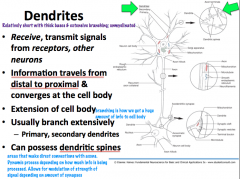
|
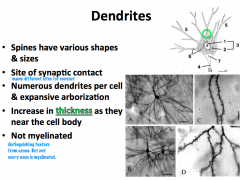
|
|
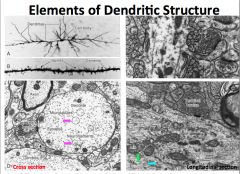
What kind of structures are present in dendrites?
|
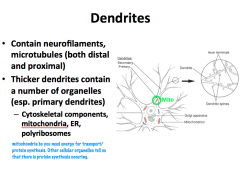
**Primary dendrites have the same cytoplasmic characteristics as the soma** As you move distally, you find less organelles; therefore, **secondary dendrites and distal branches have only cytoskeletal components**
|
|
|
|
What is the function of neuron receptors?
|
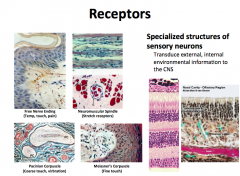
|
|
|
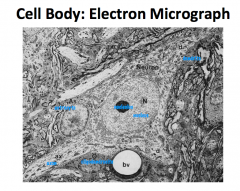
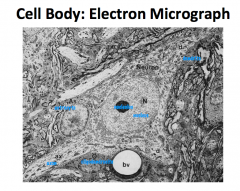
What are some characteristics of a neuron cell body?
|
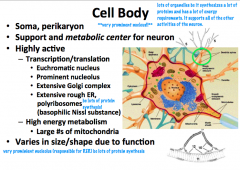
Metabolic and support center of the neuron.
Large, round, euchromatic nucleus with a prominent nucleolus. Lots of organelles, which is typical of such an actively synthetic and metabolic cell. Cell body is only like 1% of the entire volume of the nerve cell. |
|
|
What is a Nissl Substance
|
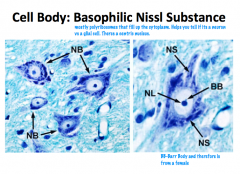
Polyribosomes, free ribosomes, and RER stain basophilic ally and appear as nissl bodies (the grainy, blue staining patterns.
The axon hillock is the only part of the cell body that is devoid of nissl bodies. |
|
|
Neuron Microtubules
|
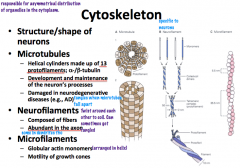
Microtubules have the *largest diameter*
they are polar with a positive and negative end and are in a dynamic state. They are shaped like helical cylinders which are made of 13 lengthwise running *protofilaments* which are composed of pairs of linearly arranged alpha and beta subunits of *tubulin* α and β tubulin → protofilament x 13 → helical cylinder |
|
|
|
Neurofilaments
|
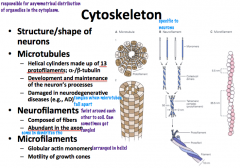
are the *most abundant* cytoskeletal component and have an *intermediate* diameter.
They are shaped of complexes of coiled fibers. 2 monomers are coiled together to form a *coiled-coil heterodimer*. 2 dimers complex together to form a tetrameric *protofilamen*. 2 protofilaments form a *protofibril*. 3 *protofibrils* are helically twisted to form the neurofilament. From smallest to largest: monomer x 2 → coiled-coil heterodimer x 2 → protofilament x 2 → protofibril x 3 → neurofilament |
**Neurofilaments are modified in Alzheiemer's disease and form Neurofibrillary tangles**
|
|
|
What do axons do? What do they contain? What do they NOT contain?
|
|
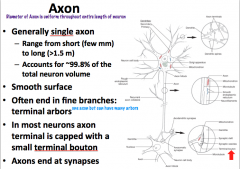
|
|
|
What is the Axon Hillock?
What is the Initial Segment? |
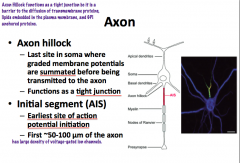
The axon hillock has **NO nissl bodies!!** (unlike cell body) Also it is NOT the site of action potential initiation!!!**
**AP initiation is in the INITIAL SEGMENT of the axon!!! -initial segment is non-myleinated! Both the hillock and initial segment have the highest density of voltage gated Na+ channels. |
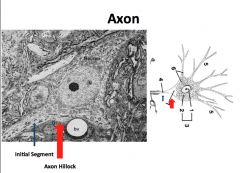
|
|
|
Myelination Patterns of the Axon
|
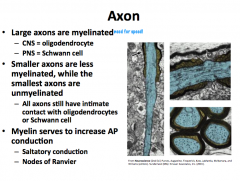
|
|
|
|
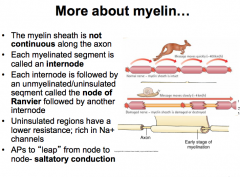
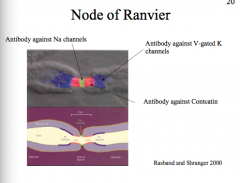
Node of Ranvier
|
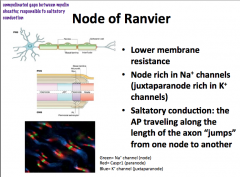
The subdomains comprising the myelin and nodes are the node itself, followed by the Paranode, and then the Juxtanode.
The Internode is between two juxtanodes and represents the area of axon completely ensheathed by myelin |
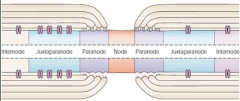
Pattern:
INTERNODE -- Juxtanode -- Paranode --NODE-- Paranode--Juxtanode -- INTERNODE -- Juxtanode -- Paranode -- NODE The Node has a lot of voltage gated Na+ channels. The Paranode has lots of Caspr1, which is the protein that attaches to the glial cell. **Glial cells attach to axons at paranodes** The juxtanode has a lot of voltage gated K+ channels |
|
|
What is an Action Potential? What is the sequence of channel openings/closings? How is it propagated?
|
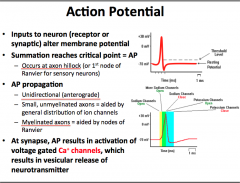
|
|
|
|
What is a Synapse? What are the components?
|
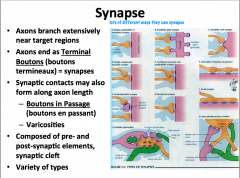
Remember that synapses can be made between axons and other dendrites, somas, or axons.
|
|
|
|
Axonal Protein Synthesis
|
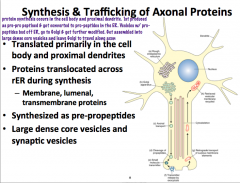
|
Because we know that the axon lacks the machinery for protein synthesis, it must get its proteins from the soma and proximal dendrites via transportation mechanisms.
Most membrane, lumenal, and secretory proteins are synthesized as prepropeptides, cleaved into pro peptides, and packaged into large dense core vesicles or synaptic vesicles in the ER. **Large dense core vesicles contain peptides! Synaptic vesicles contain neurotransmitters!** After modification in the golgi, they are transported via axonal transport. In the nerve terminal, pro peptides are cleaved into peptides and presynaptic vesicles are assembled (essentially just repackaged) |
|
|
Axonal Transport: What is it important for? What are the types?
|
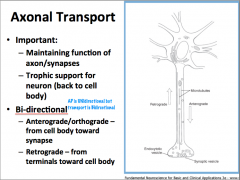
axonal transport can be anterograde (toward the axon terminal) or retrograde (away from the axon terminal)
|
|
|
|
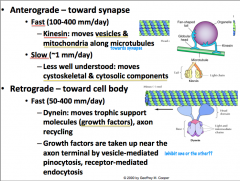
Anterograde Transport
|
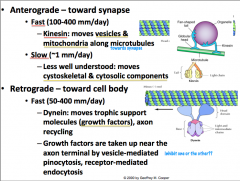
Can be slow or fast.
Slow anterograde transport is not well understood, but is reserved for components of the cytoskeleton (i.e. actin) and cytosolic proteins (i.e. calmodulin). Fast anterograde transport involves using a MICROTUBULE as a stationary track and KINESIN, an ATPase, as a 'train' that carries vesicles destined towards the terminal. The kinesin of course is intimately associated with mitochondria due to its ATPase activity. |
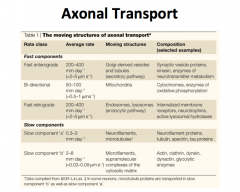
|
|
|
Retrograde Transport
|
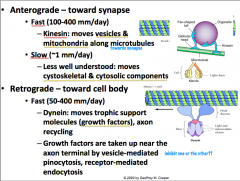
Is only fast, so it is called "fast retrograde transport".
It is not as fast as anterograde, but it is still relatively fast. Along with recycled synaptic and dense core vesicles, trophic factors are transported from terminal to cell body. The items that are being transported here are also vesicularly mediated by either endo- or pino-sytosis. Like anterograde transport, MICROTUBULE is used as a stationary tract, but this time DYNEIN is what drives the vesicles up to the cell body **CLINICAL IMPLICATION: HERPES, POLIO, RABIES, TETANUS TOXINS, AND OTHERS ARE TRANSPORTED VIA RETROGRADE TRANSPORT** |
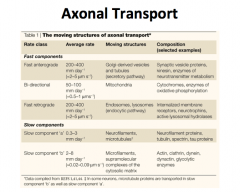
|
|
|
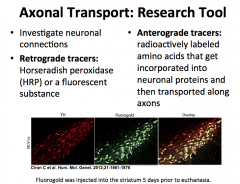
How are viruses transported in axons?
|
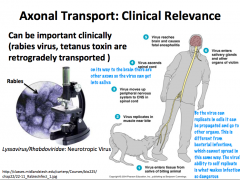
**CLINICAL IMPLICATION: HERPES, POLIO, RABIES, TETANUS TOXINS, AND OTHERS ARE TRANSPORTED VIA RETROGRADE TRANSPORT**
|
|
|
|
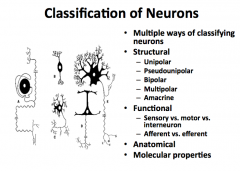
Name some ways you can classify neurons
|
|
|
|
|
Structural Classification of Neurons
|
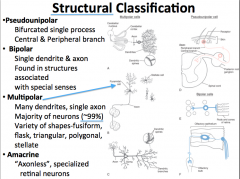
Polarity is used to describe the pattern of the extensions of the cell body.
Bipolar neurons have a single axon and only one primary dendrite. Pseudounipolar has a single process that bifurcates proximal to the soma. Multipolar have many primary dendrites and a single axon. These are typical of the majority of cells. Anacrine are a special type of neuron that have only dendrites. They are only found in the retina. |
|
|
|
Functional Classification of Neurons
|
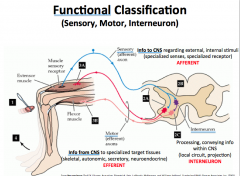
|
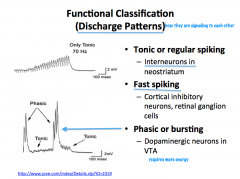
|
|
|
Anatomical and Molecular Classification of Neurons
|
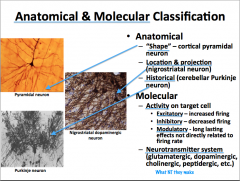
|
|
|
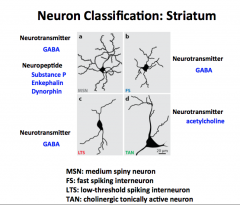
Just stare at this… come back to it when new MNTS are posted to see what its all about.
|
Come back to this later.
|
|
|
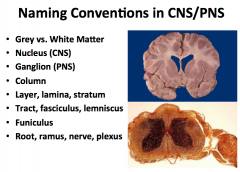
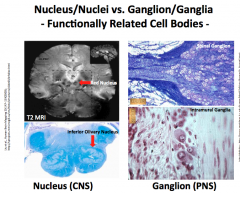
In the CNS:
-What do you call a group of cell bodies? -What do you call a bundle of axons? -What do you call a bundle of parallel tracks? In the PNS: -What do you call a group of cell bodies? -What do you call a bundle of axons? |
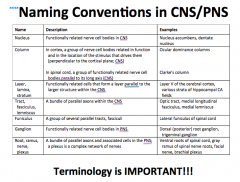
MEMORIZE THIS!
|
|
|
What are some other words used to describe CNS cell body organization?
|
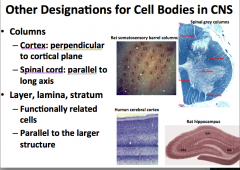
"Columns"
-in the spinal cord, columns of cell bodies are arranged parallel to the length of the spine. Think of them stacked up like a stack of coins. -In the cortex, columns of cell bodies are arranged perpendicularly to the cortical plane which is essentially the same pattern that they are arranged as in the spine, except that the stacks of coins follow the contour of the surface of the brain |
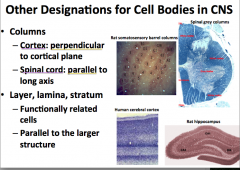
"Layer, Lamina, and Stratum"
-used to describe functionally related cell bodies that are arranged in parallel to the larger structure |
|
|
Tracks in the CNS vs. PNS
|
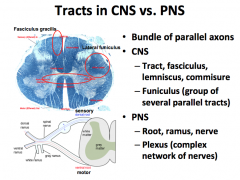
|
|
|
|
Whats the difference between the following:
-nerve -neuron -nerve fiber -neurites -neuropil |
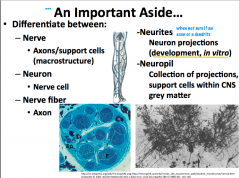
|
|
|
|
Peripheral Nerve Components
|
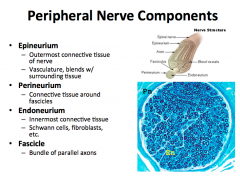
An individual axon is surrounded by endoneurium.
A bundle of axons, called a fascicles, is ensheathed by perineurium. A bundle of fascicle are ensheathed by the epineurium. -this epineurium is the outer most connective tissue that defines the nerve, contains the vasculature, and blends with surrounding tissue. |
|
|
|
Degeneration and Regeneration of Nerve Tissue
-What happens if the cell body dies? -What happens if the axon is damaged? |
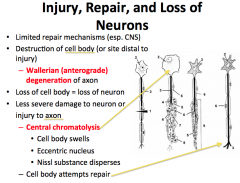
Neurons no longer divide.
When a Cell Body dies, the neuron cannot survive. Axonal damage is more interesting… The point distal from which damage occurs cannot get support from the cell body and is said to go through WALLERIAN DEGENERATION. -if the damage affects the cell body, CHROMATOLYSIS occurs. This is evidenced by swelling, distribution of nissl substance, and an eccentrically located nucleus. The neuron often degenerates. |
|
|
|
What is Wallerian Degeneration?
|
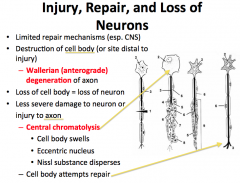
Axonal damage is more interesting… The point distal from which damage occurs cannot get support from the cell body and is said to go through WALLERIAN DEGENERATION.
-if the damage affects the cell body, CHROMATOLYSIS occurs. This is evidenced by swelling, distribution of nissl substance, and an eccentrically located nucleus. The neuron often degenerates. |
|
|
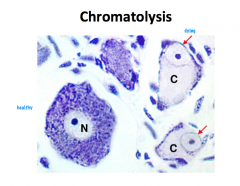
What is Chromatolysis?
|
|
|
|
|
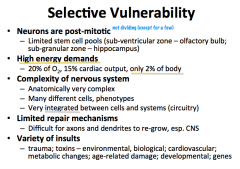
What makes neurons so vulnerable?
|
|
|
|
|
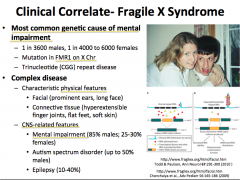
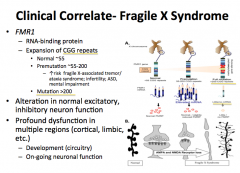
Fragile-X Syndrome:
-What is it? -What are the characteristics? -What kind of mutation causes it? |
|
|
|
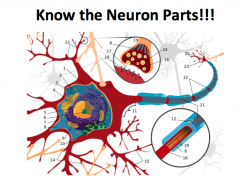
Label the parts!
|
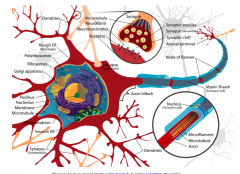
|
|
|
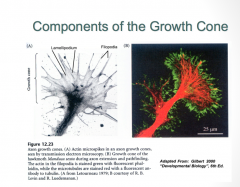
The Growth Cone Structure
|
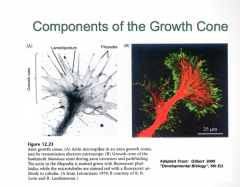
The growth cone is composed of 2 primary components:
-Filopodia (green): are constantly being turned over, and their primary job is to survey the environment for signaling factors which determine the pattern of axon growth -Lamelipodia (red) |
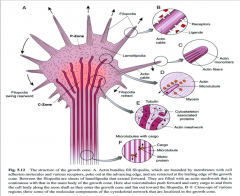
A. Actin bundles fill filopodia, which are bounded by membranes with cell adhesion molecules and various receptors, poke out at the advancing edge, and are retracted at the trailing edge of the growth cone. Between the filopodia are sheets of lamellipodia that extend forward. They are filled with an actin meshwork that is continuous with that in the main body of the growth cone. Here also microtubules push forward and carry cargo to and from the cell body along the axon shaft as they enter the growth cone and fan out toward the filopodia.
B-F. Close-ups of various regions show some of the molecular components of the cytoskeletal network that are localized in the growth cone. |
|
|
3 Domains of the Growth Cone
|
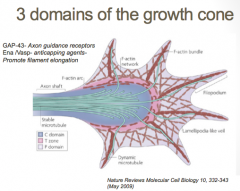
The growth cone is described in terms of three regions: the peripheral (P) domain, the transitional (T) domain, and the central (C) domain. The peripheral domain is the thin region surrounding the outer edge of the growth cone. It is composed primarily of an actin-based cytoskeleton, and contains the lamellipodia and filopodia which are highly dynamic. Microtubules, however, are known to transiently enter the peripheral region via a process called dynamic instability. The central domain is located in the center of the growth cone nearest to the axon. This region is composed primarily of a microtubule-based cytoskeleton, is generally thicker, and contains many organelles and vesicles of various sizes. The transitional domain is the region located in the thin band between the central and peripheral domains.
|
|
|
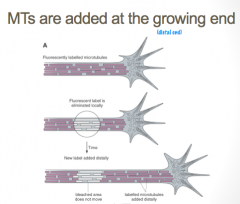
Growth Cone Elongation
|
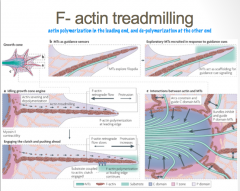
F-actin is characterized by polymerization, or the addition of new actin monomers, at its leading edge, and loss of monomers at its tail end. This dynamic process is known as "tread milling"
|
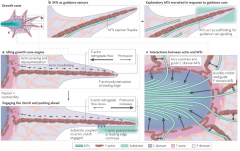
a | Together, filamentous (F)-actin treadmilling (in which F-actin is polymerized at the leading edge and severed at the transition (T) zone, with the subunits recycled back to the leading edge) and F-actin retrograde flow (the continuous movement of F-actin from the leading edge towards the centre of the growth cone) keep the growth cone engine idling. When retrograde flow and polymerization forces are balanced, no protrusion occurs. When filopodia encounters an adhesive substrate, growth cone receptors bind to the substrate and are coupled to F-actin through 'clutch' proteins. This engages the clutch, anchoring F-actin with respect to the substrate and attenuating F-actin retrograde flow. Further F-actin polymerization pushes the membrane forward, which results in growth cone protrusion. b | Peripheral (P) domain microtubules (MTs) explore filopodia along F-actin bundles and might act as guidance sensors. As a filopodium encounters a guidance cue, exploratory MTs might act as scaffolding for further signalling, and additional MTs are recruited to the region. c | Actin has a role in determining MT localization in the growth cone. Actin arcs constrain and guide central (C) domain MTs (single arrows), and F-actin bundles inhibit and guide P domain MTs (double arrows).
|
|
|
Growth Cone Turning
|
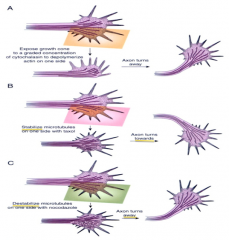
Actin and microtubules steer growth cones.
(A) local depolymerization of actin on one side of a growth cone causes it to turn the other way. (B) local stabilization of microtubules on one side of a growth cone causes it to turn towards that side. ( C) Destabilization of microtubules on one side causes it to turn the other way. |
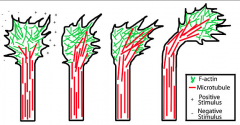
|
|

How do neurons know where to go?
|

There are 4 classes of axonal guidance molecules: Soluble and Bound molecules, and attractive and repulsive.
Attractive molecules inhibit retrograde flow of the actin filaments and promote their assembly |
Growth cones contain receptors that recognize these guidance cues and interpret the signal into a chemotropic response. The general theoretical framework is that when a growth cone "senses" a guidance cue, the receptors activate various signaling molecules in the growth cone that eventually affect the cytoskeleton. If the growth cone senses a gradient of guidance cue, the intracellular signaling in the growth cone happens asymmetrically, so that cytoskeletal changes happen asymmetrically and the growth cone turns toward or away from the guidance cue
|
|
|
What are the 2 criteria required for axon guidance molecules?
|
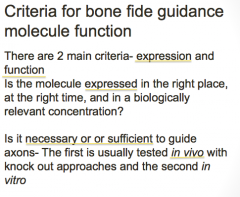
|
|
|

Role of Netrin and Robo/Slit in the development of bilateral nervous systems.
|

|
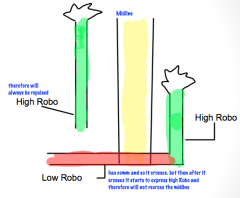
|
|
|
Sema-3A as an Axon Guidance Molecule
|
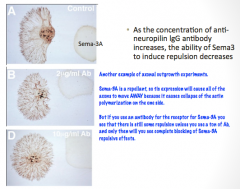
|
|
|
|
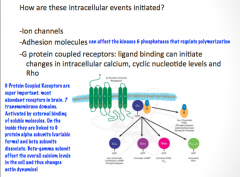
Name 3 important intracellular pathways in axonal guidance
|

|
|
|
|
What role does calcium play in axonal guidance?
|

|
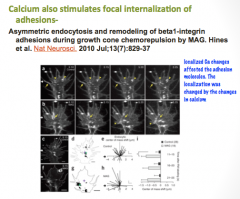
|
|
|
What role do cyclic nucleotides play in axonal guidance?
|

|

|
|
|
What role to Rho GTPases play in axonal guidance?
|
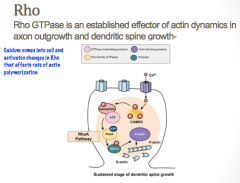
|
|
|
|
Do axonal guidance molecules play a role in the mature CNS?
|
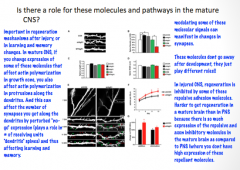
|

|
|
|
What is synaptic transmission?
|
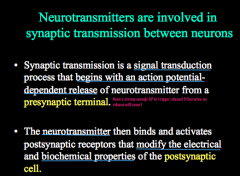
|
|
|
|
In what ways can pre and post synaptic connections be variable?
|
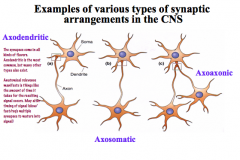
|
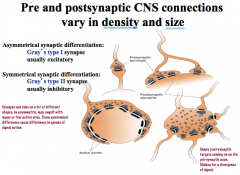
Not always a one-to-one construction!
4 main types… -simple: one presynaptic element goes to one postsynaptic element -it can bifurcate before reaching the target to have a greater signal effect -it can be a single presynaptic element with many active sites where NTs are released -you can have multiple synapses from different sources coming to one neuron |
|
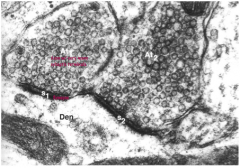
Criteria for identifying a NT (6)
|
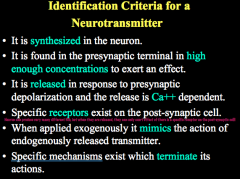
|
|
|
|
How can we categorize NTs by chemical structure?
|
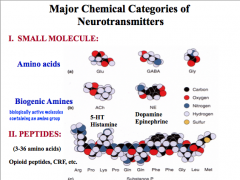
|
|
|
|
How can we categorize NTs by function?
|

|
|
|
|
How are small molecule NTs synthesized?
|
Enzymes for small molecule NT synthesis are formed in the cell body of the neuron and transported down to the presynaptic terminal where they synthesize the NTs. NTs are then stored in vesicles until they are released into the synaptic cleft to bind their receptors on the postsynaptic terminal.
|
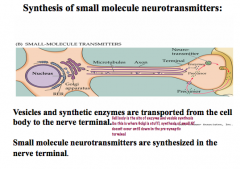
|
|
|
How is ACh synthesized?
|
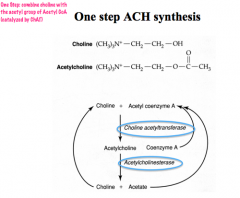
|
|
|
|
What is the life cycle of ACh?
How is the signal terminated? |
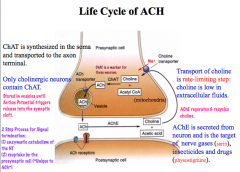
Termination: ACh is metabolized into choline and acetate by AChE. Choline is then taken up from the synaptic cleft. AChE is the target of nerve gases and insecticides.
|
|
|
|
Where are the ACh (cholinergic) nuclei in the CNS? Where do they project to?
|
ACh is created in 8 nuclei in the basal forebrain and the upper brain stem. They project to the hippocampus, thalamus, neocortex, and cortex.
|
|
|
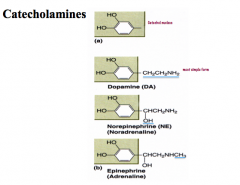
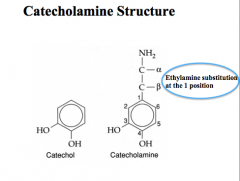
How are catecholamines synthesized?
|
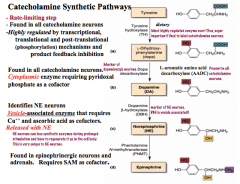
Precursor: Tyrosine
Rate Limiting Step: TH CNS has distinct neurons for all 3 catecholamines Immunohistochemical techniques (i.e. fluorescent antibodies) for synthetic enzymes with identify which type of neuron you are looking at |
|
|
|
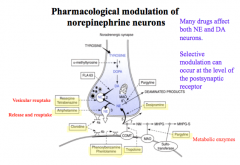
How are catecholamine signals terminated?
|
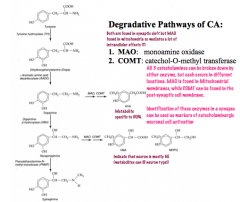
|
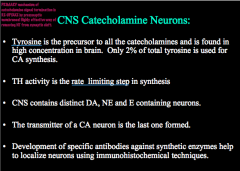
|
|
|
Life Cycle of Norepinephrine
|
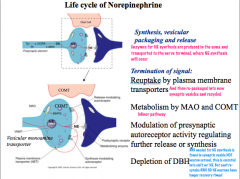
NE has 2 additional methods of regulation/termination- auto receptors exist on noradrenergic neurons to regulate release, and DBH depletion due to release with NE.
|
|
|
|
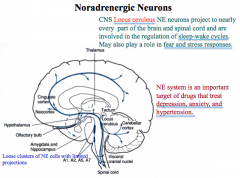
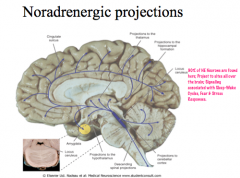
Where are NE nuclei located in the CNS?
|
|
|
|
|
Where are Dopamine nuclei located in the CNS?
|
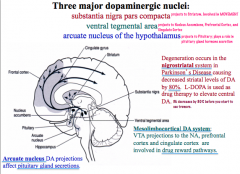
|
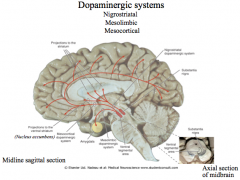
|
|
|
How is Serotonin synthesized?
How can serotonin neurons be pharmacologically modulated? |
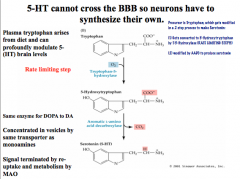
|
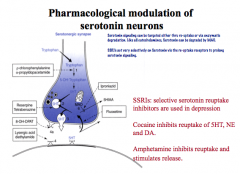
|
|
|
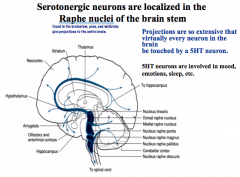
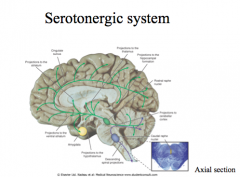
Where are Serotonin nuclei located in the CNS?
|
|
|
|
|
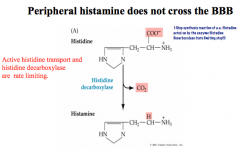
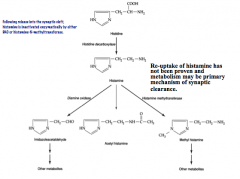
How is Histamine synthesized?
How is its signal terminated? |
|
|
|
|
Where are Histamine nuclei located in the CNS?
|
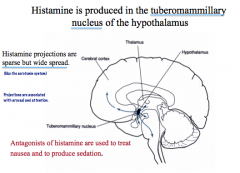
|
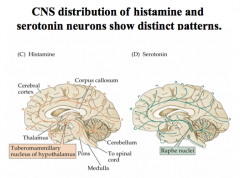
|
|
|
What are the 2 main categories of amino acid neurotransmitters?
|

|
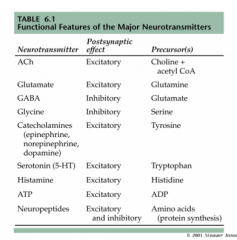
|
|
|
What is the life cycle of excitatory glutamate neurons?
What kind of pathophysiological and physiological processes is it involved in? |
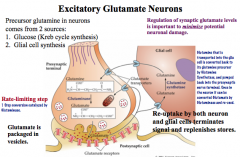
|

|
|
|
What is the life cycle of inhibitory GABA neurons?
|
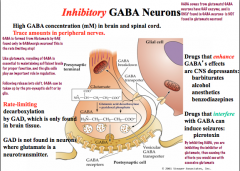
|
|
|
|
What role to Glia play in glutamate and GABA neurons?
|
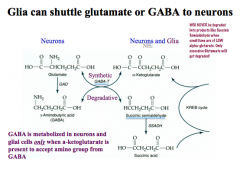
|
|
|
|
How do Glycine inhibitory neurons differ from GABA neurons?
|
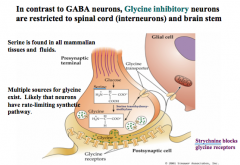
|
|
|
|
How are peptide NTs synthesized and packaged? What else is unique about the life cycle of peptide NTs?
|
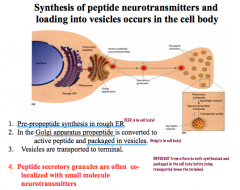
|
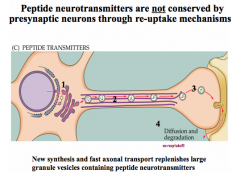
|
|
|
Describe the frequency dependence of peptide and small molecule NT release
|
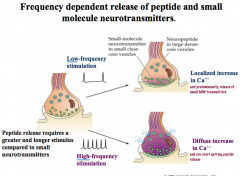
|
|
|
|
What kind of projections do monoamines have?
|
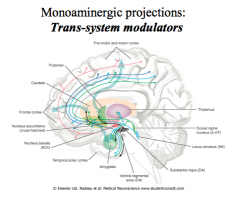
|
|
|
|
What kind of modulators are neuropeptides?
|
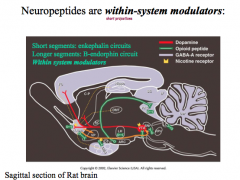
|
|
|
|
Define the following:
-Receptor -Binding Site -Ligand -Agonist -Antagonist -Allosteric Modulation |

|
|
|
|
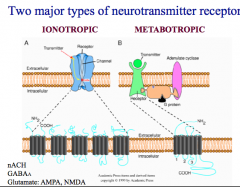
What is the difference between Ionotropic and Metabotropic Receptors?
|
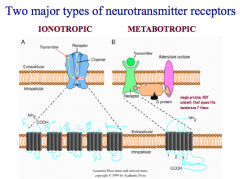
|
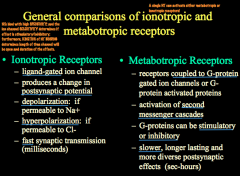
|
|
|
What are the 2 ways an ion channel can be opened?
|
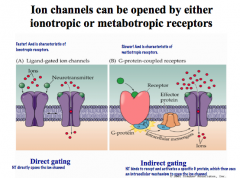
|
|
|
|
What are the general principles guiding specificity, kinetics, and binding of NTs to receptors?
|

|
|
|
|
What are the 7 Ionotroptic receptors for ACh, Glutamate, Serotonin, GABA, and Glycine?
What ions pass through them? |
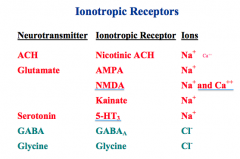
|
|
|
|
What are the 2 major families of ionotropic receptors?
How are they different? |
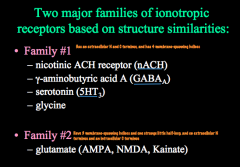
Family 1 looks like nicotinic ACh receptors
Family 2 includes all the subtypes of glutamate receptors. Both families have 4-5 subunits composing a pore. -Family 1 has 4 membrane spanning domains -Family 2 has only 3 membrane spanning domains, and one domain that doesn't cross (called a "pore loop") |
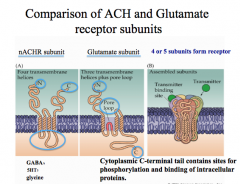
|
|
|
How do ionotropic receptor subunits vary?
|
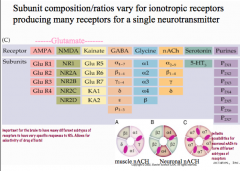
Each receptor has numerous subunits that can be combined to form a receptor.
For nicotinic ACh receptors, including gamma and delta subunits with alpha and be a subunits creates a muscular nicotinic ACh receptor, while using varying combinations of alpha and beta subunits creates a neuronal nicotinic ACh receptor. This concept of combining varying subunits to create varying receptors applies to GABA and glycine receptors, but NOT to glutamate receptors. -for glutamate receptors, the subunits listed under subtypes (AMPA, NMDA, and Kainate) are the only subunits that form each receptor. |
|
|
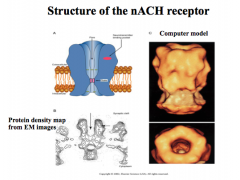
Describe the structure and characteristics of nicotinic ACh receptors
|
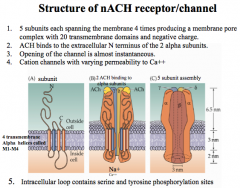
5 subunits, and are negatively charged. Thus, allowing only Na+ and a little Ca++ through, depolarizing the cell and sending a stimulatory signal.
Every functional nicotinic ACh receptor must have 2 subunits to open the channel, while the other 3 subunits can vary. |
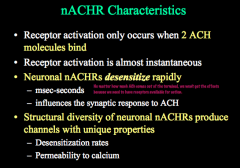
This channel opens very quickly and also desensitizes very quickly, but how fast these rates are and their permeability to Ca++ depends on which subunits are present.
|
|
Describe the structure and characteristics of GABA receptors
|
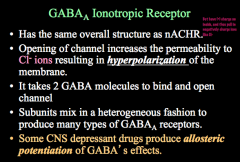
Pore is positively charged! This allows Cl- to enter the cell, hyper polarize it, and send an inhibitory signal.
Subunits can mix to form varying types of GABA receptors and it takes 2 GABA molecules to open this receptor |
|
|
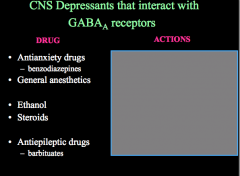
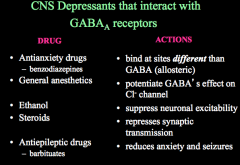
How can CNS depressant drugs affect GABA receptors?
What actions do the above drugs have on GABA receptors? |
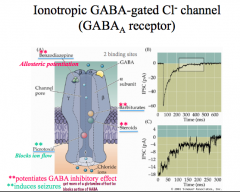
CNS depressants act to allosterically potentiate GABAs effects, meaning that these drugs will keep GABA receptors open and inhibit and neuron that has this receptor on it.
Remember, allosteric sites do not interfere with the binding site and thus are NOT antagonists! If you block ion flow in a GABA receptor (picrotoxin), you allow glutamate signaling to go unchecked and this results in seizures! |
|
|

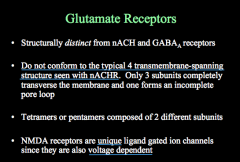
Describe Glutamate Receptors
|
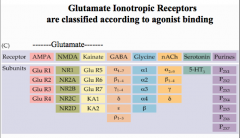
Have only 3 membrane spanning domains, and consist of 2 types of subunits forming a 4-5 subunit structure.
There are 3 types of glutamate receipts: AMPA, NMDA, and Kainate |
|
|
|
AMPA Receptors vs. NMDA Receptors
|

AMPA receptors have fast kinetics and desensitize rapidly. They are permeable to Na+ and K+, but NOT to Ca++.
|
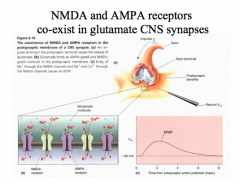
Both are typically found on a postsynaptic glutamate neuron.
AMPA receptors open their channels first, partially depolarizing the cell. With glutamate and glycine present, NMDA channels will then open, allowing depolarization and Ca++ influx into the cell. While AMPA receptor signals are small and transitory, NMDA receptor signals are much longer and larger. |
|
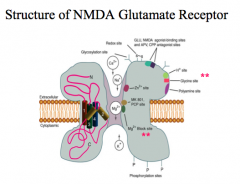
Describe the structure of NMDA receptors.
What kind of processes are NMDA receptors involved in? |
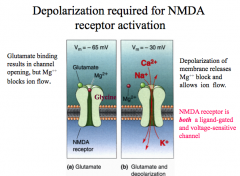
Unique because they are both ligand gated AND voltage dependent!**
A glutamate and a glycine are required to open the channel and depolarization (at least -30mV) is required to remove the Mg++ that blocks the pore to allow Na+, K+, and Ca++ to flow through the channel. Overall, this channel has slower kinetics than AMDA channels. |

|
|
|
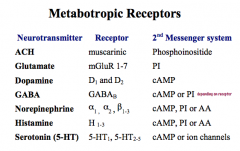
What are some characteristics of Metabotopic Receptors?
|
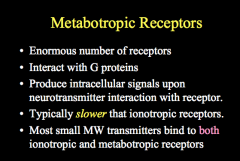
Most metabotropic receptors use G-proteins to cause their signaling, leading them to be considered to have indirect gating, as any ion channels that are opened are done thru G-protein signaling.
Often, these G-proteins also activate 2nd Messenger Systems and signaling cascades. This signaling is slower than ionotropic receptors BUT their effects last longer. Typically, these receptors have 7 membrane spanning domains, and can cause both inhibitory and stimulatory signals. Remember, these receptors can be signaled by many of the same NTs that work on ionotropic receptors. |
|
|
|
Role of G-proteins in Metabotropic Receptors
|
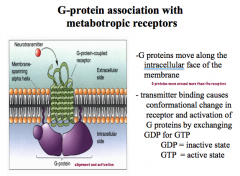
Once activated by a NT, G-proteins can move along the internal surface of the plasma membrane, causing many different effects!
To move, the binding causes a conformational change in the receptor that allows for an exchange of GDP for GTP. -Without GTP, G-proteins are in their inactive state. |
|
|
|
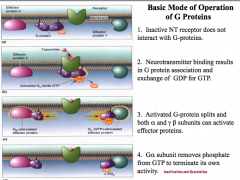
What are 2 pathways that G-proteins can use to cause changes in the cell?
|
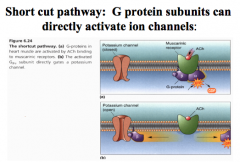
(1)can directly interact with ion channels, opening or closing these channels to change the membrane charge, causing depolarization or hyper polarization
|
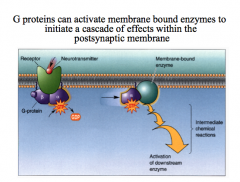
(2) activation of 2nd messenger systems, like cAMP
|
|
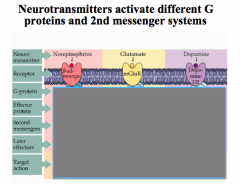
|
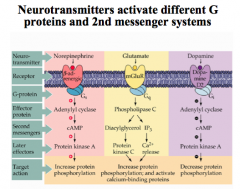
Note that NE and Glutamate activate a stimulatory cascade, while dopamine activates an inhibitory cascade.
|
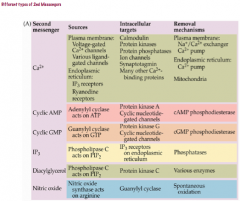
|
|
|
|
|
|
|
How can different subtypes for a NE receptor cause different effects?
|
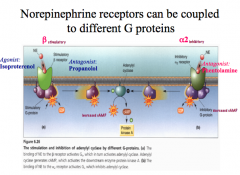
For NE, a β receptor stimulates a cAMP cascade (a G-stimulatory protein), while an α2 receptor inhibits a cAMP cascade (a G-inhibitory protein).
Both receptor subtypes bind the same neurotransmitter, but their effects are very different! |
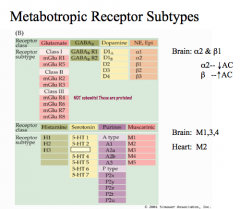
|
|
|
What does metabotropic receptor response depend on?
|
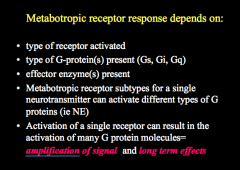
|
|
|
What is signal amplification in metabotropic receptor responses?
|
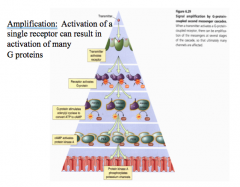
One receptor can activate many G-protein molecules, causing amplification of signals and long-term effects on the postsynaptic neurons.
Thus, while there are fewer metabotropic receptors on a cell, their effects can sat longer and be greater, by changing gene expression or by having a single G-protein cause a cascade that acts on numerous proteins within the cell for a prolonged period of time. Remember, the longer a signal is present, the more time there is to change the transcriptional patterns in a cell. |
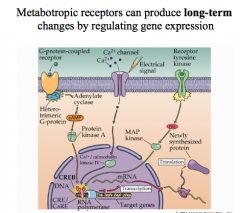
|
|
|
What are transmembrane domains?
|
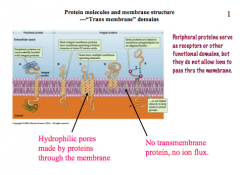
|
|
|
|
Active vs. Passive Transport
|
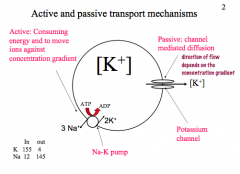
|
|
|
|
Properties of Ionic Channels
|
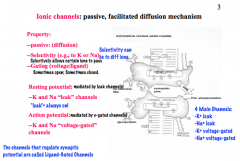
|
|
|
|
Which channels determine Resting Potential and Action Potential?
|
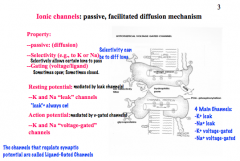
|
|
|
|
What generates membrane potential?
|
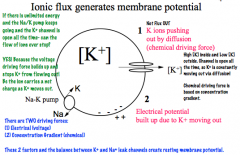
|
|
|
|
What is the charge of a nerve cell at rest?
|
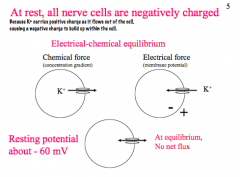
|
|
|
|
What is the Nernst equation?
|
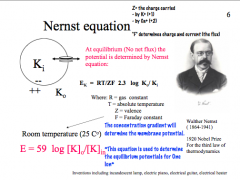
|
|
|
|
How is the resting membrane potential established?
|
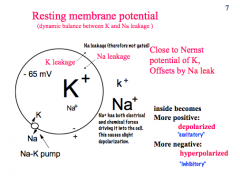
|
|
|
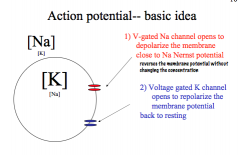
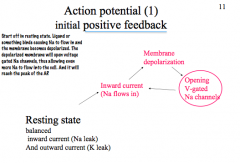
Describe Action Potential in terms of feedback loops and the channel kinetics
|
|
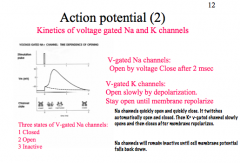
|
|
|
What determines the shape of an AP?
|
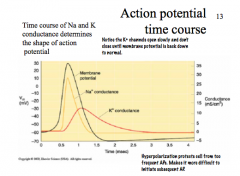
|
|
|
|
What is the difference between Absolute and Relative Refractory Periods?
|
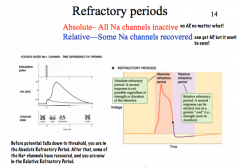
|
|
|
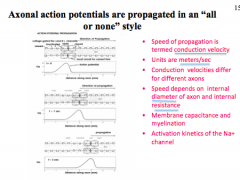
How does diameter affect AP?
|
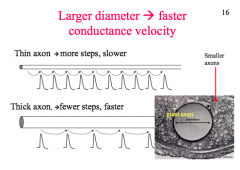
|
|
|
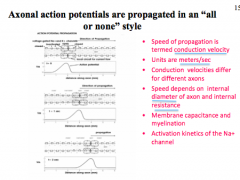
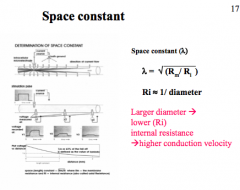
How does Space Constant affect AP?
|
|
|
|
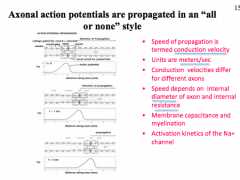
How does Capacitance affect AP?
|
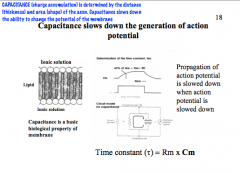
|
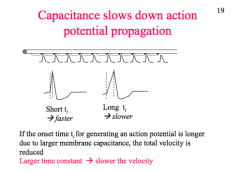
|
|
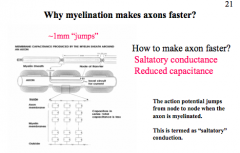
Why does myelination make axons faster?
|
|
|
|
|
What determines flux and direction?
|
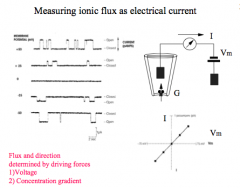
|
|
|
|
Compare the differences between the electrical synapse and the chemical synapse
|
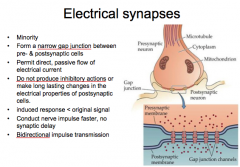
|
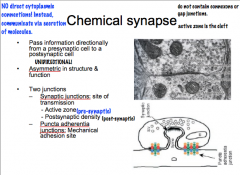
|
|
|
Describe the general structure of the electrical and chemical synapses.
|
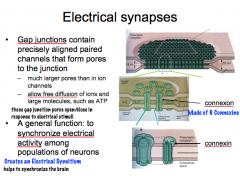
|
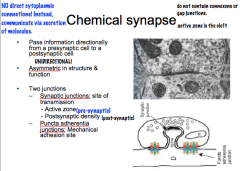
|
|
|
Discuss the 3 types of synapses and explain their similarities and differences.
|
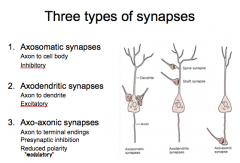
|
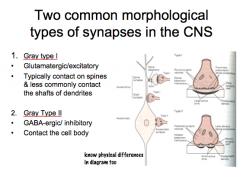
|
|
|
Discuss the components of presynaptic compartments and postsynaptic compartments and their functions.
|
1. Synaptic Vesicles
2. Large Dense Core Vesicles 3. Endocytic Organelles 4. Smooth ER 5. Mitochondria 6. Presynaptic Dense Grid |
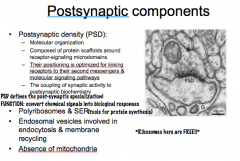
|
|
|
Discuss the classifications and functions of dendritic spines.
|
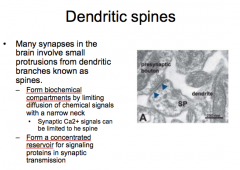
|
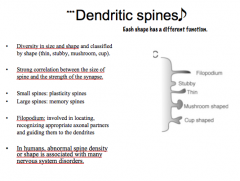
|
|
|
Describe the basic mechanisms of synaptic transmission.
|
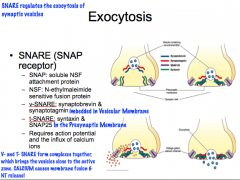
|
|
|
|
Describe Synaptic Vesicles
-How big are they? -What do they store? -What are they regulated by? -What is there signaling like? |

|
|
|
|
Describe the 3 Pools of Synaptic Vesicles
|
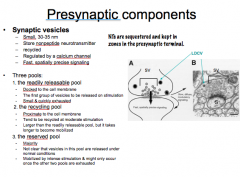
|
|
|
|
Describe Large Dense Core Vesicles
-How big are they? -What do they have inside? -Where are they synthesized? -Are they recycled? -How many are there? -Where are they located? -What is needed for their release? -How is there signaling characterized? |
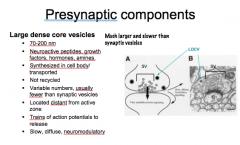
|
|
|
|
Endocytic Organelles
-What are they? -Where are they located? -How many are there? -What do they do? |
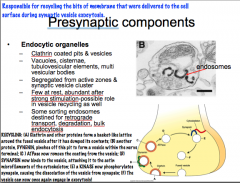
|
|
|
|
Role of Smooth ER as a Presynaptic Component
|
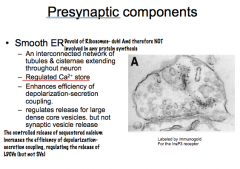
|
|
|
|
Role of the Mitochondria as a Presynaptic Component
|
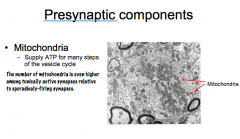
|
|
|
|
What is the Presynaptic Dense Grid?
-Where is it located? -What is it composed of? -What role does it play? |
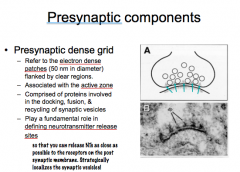
|
|
|
|
Synaptic Cleft
-What is it? -What purpose does it serve? -What kind of transmission occurs here? |
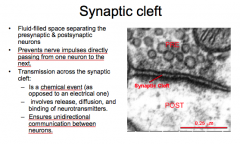
|
|
|
|
What is the Postsynaptic Density (PSD)? What does it do?
|
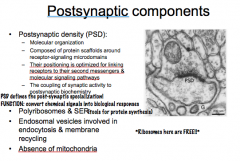
|
|
|
|
EPSPs vs. IPSPs
|
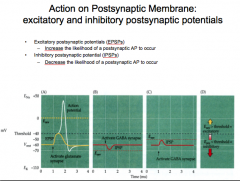
|
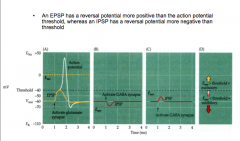
|
|
|
What determines Reversal Potential?
|
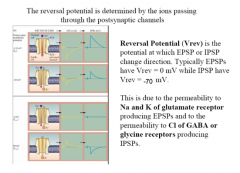
|
|
|
|
Summation of Synaptic Potentials
|
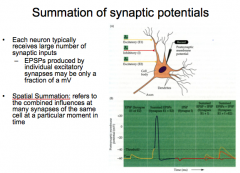
|
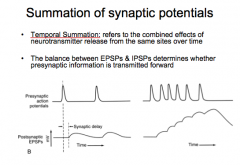
|
|
|
What is Ubiquitination?
|
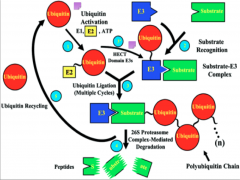
The tagging of a substrate (usually a protein) with one or several Ub molecules.
In the ubiquitin-proteosome system, Ub tags target proteins with Ub so they can be identified by the proteosome for degradation. This is accomplished by an enzymatic cascade ending with Ub ligase dragging the substrate to the proteosome. NOTE: Ubiquination has lots of other functions- like it can be used in post-translational modification, aiding proteins in exiting the nucleus or docking to the cell membrane, and even to activating newly synthesized mitochondria. |
|
|
|
Ub-E1/ "Activating Enzyme"
|
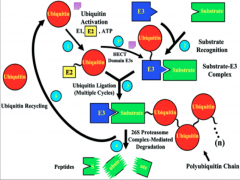
E1 serves to activate the pathway, but E1 is also the Ub molecule itself.
It starts the pathway off by activating E2 by adding an ATP (remember this process requires a lot of energy) |
|
|
|
Ub-E2/ "Conjugating Enzyme"
|
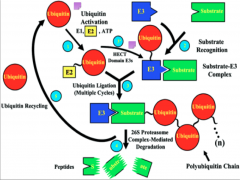
E2 is a transitional enzyme. Once it is activated by E1, it forms a transient bond with the Ub and transfers it to E3.
Why is it needed if all it does is hand over the Ub? Ubiquitination has a variety functions in the cell, and the function that is enacted is partly determined by the level of ubiquitination. E2 helps determine how much ubiquitination takes place |
|
|
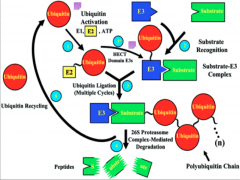
Ub-E3/ "Ub Ligase"
|
It is the most important of the enzymes for several reasons:
1. E3 is the enzyme that recognizes and binds to the target protein. 2. E3 forms a covalent bond with E1, which is very hard to break. The bonds formed in the E1 and E2 steps are not covalent and easily broken. 3. The proteosome recognizes only E3, thus E3 is responsible for dragging the ubiquitinated target protein to the proteosome. 4. E3 is *species specific*. There are different versions of E3 that result in different pathways |
|
|
|
HECT domain E3s
|
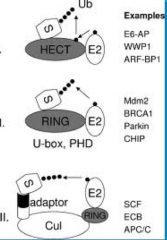
- HECT is very important in invertebrates, species like yeast and c. elegans
-This E3 *forms a transient thirster bond with the Ub * from the E2 before *transferring it to the target protein |
|
|
|
RING domain E3s
|
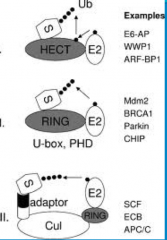
-RING is important in vertebrates (us!)
-These E3 enzymes act as a molecular scaffold. They bring the Ub-E2 complex in close proximity, resulting in an *isopeptide bond joining the Ub DIRECTLY to the protein* -A problem with RING is a strong candidate for what is going wrong in almost every disease where you have failure of ubiquitination or failure of degradation of ubiquitinated proteins. |
|
|
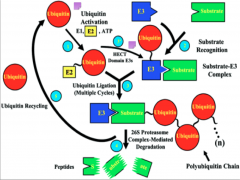
Mechanism of Ubiquitination
|
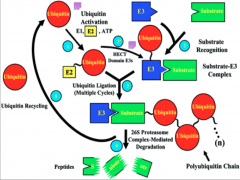
Here you have E1 (depicted as a Ub molecule) starting off in the upper right of the diagram. The process begins as it activates and binds to E2 (the conjugating enzyme).
From this point E2 can undergo one of two pathways, depending on the domain of E3 present. In the case of the HECT domain, a thiester bond forms between E3 and Ub, and subsequently the Ub is transferred to the substrate. If a RING E3 is present, the Ub is attached directly to the protein via an isopeptide bond, and at no point forms a bond with E3 itself. At this point the target protein is tagged and carried to its final destination. All of these processes have the potential to go to the 26s proteosome for degradation. Failure of any of these steps to occur results in failure of ubiquitination. |
|
|
|
What must always be present to tag a protein with a Ub molecule?
|
A Lysine residue!
Without a Lysine residue, it is impossible to tag a target protein with a Ub molecule! |
|
|
|
What are the different levels of ubiquitination?
-Mono -Poly -Multi |
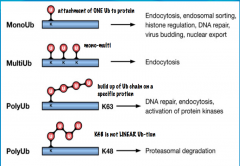
Mono: the E3-substrate complex is only tagged with one Ub.
Poly: when E2 undergoes multiple cycles, it is able to attach several Ub molecules to the E3-substrate complex. This can result in a Ub chain. Multi: this is a type of ubiquitination where multiple branches or Ub chains are seen. For example there may be one chain of Ub molecules coming off the target protein with several additional Ub chains branching off. |
|
|
|
Which lysine residues are involved in the types of Ubiquitin chains? What are their functions?
|

A lysine residue is always necessary to bind a Ub. 7 lysine residues on Ub molecules contribute to chain formation for cell signaling: lys 6, lys 11, lys 27, lys 29, lys 33, lys 48, and lys 63
Any of the lysine residues can be involved in mono-ubiquitination. Ubiquitination and function are also cell-specific. For example, in the brain, mono-ubiquitination may be important for degradation of proteins, while it may function in histone regulation in cells of the kidney. |
|
|
|
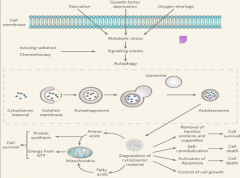
Autophagy
|
"self-eating"
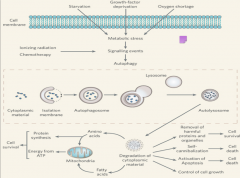
In the case that the cell needs to degrade substances such as proteins that are too big for the proteosome such as carbohydrates, lipids, and defective organelles, the autophagy-lysosome system comes into play. Autophagy has different physiological roles, as evidenced by the several different stimuli that can induce it. Among these are: starvation, growth factor deprivation, oxygen shortage, ionizing radiation, starvation, and necrosis. All of these cause metabolic stress and can lead to increased rates of autophagy. |
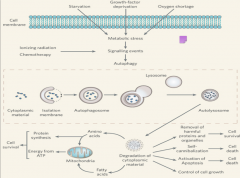
|
|
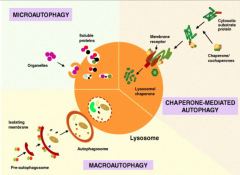
What are the 2 forms of autophagy?
|
1. Macroautophagy: involves degradation of proteins, broken ER, lysosomes, carbohydrates and other large particles.
2. Mitophagy: specific degradation of the mitochondria. |
|
|
|
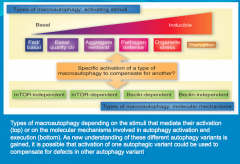
What role do mTOR and Beclin play in autophagy?
|
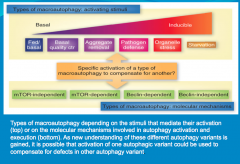
Can induce autophagy.
Either one, or both of these molecules, can induce autophagy. But this is tricky because they do not always behave this way. Because of this variability, autophagy an be mTOR-Dependent (mTOR detected), mTOR-Independent (mTOR not detected), and similarly Beclin-Dependent, or Beclin-Independent. |
|
|
|
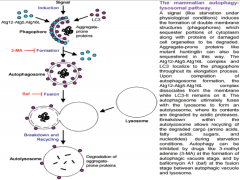
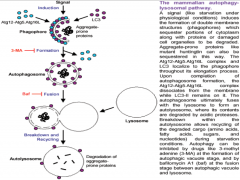
What are the 3 steps in Autophagy?
|
1. Phagophore formation, ATG activation via ubiquitination and LC3 activation.
2. LC3-1 conversion to LC3-2 via lipidation. Maturation of phagophore into an autophagosome. 3. Fusion process, degradation. |
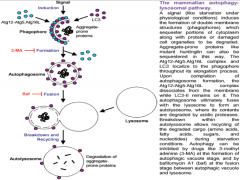
|
|
Autophagy Step 1
|
Ubiquitination drives autophagy.
This process begins when accumulating substances within the cell are engulfed by cellular membrane, creating a vesicle. This vesicle is called the PHAGOPHORE. It is though that with neurons and other cells (excluding glia and immune cells) that the membrane making up this vesicle comes from the ER. The phagophore will continue to mature with the help of 2 important molecules in its membrane, (ATGs (Autophagy-like Genes) 12 and 15. As the vesicle closes around the internal depris, ATG 12 and 15 are activated via ubiquitination, and covalently bond. They additionally bond with, and begin to activate nearby molecule LC3 (Light Chain protein) |
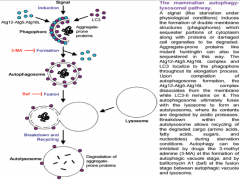
|
|
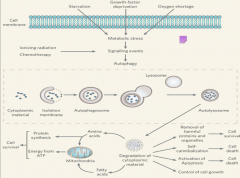
Autophagy Step 2
|
As LC3 becomes activated, it anchors to the vesicle cell membrane, both on the internal and external surfaces.
The LC3 is known as LC3-1 up to this point. It is now lipidated and known as LC3-2. At this point LC3 is completely activated, resulting in the *loss of ATG 12 and 15* from the vesicle membrane. All of these events together result in the maturation of the phagophore into an *AUTOPHAGOSOME* |
|
|
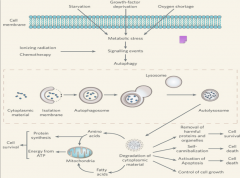
Autophagy Step 3
|
In the final step, the autophagosome fuses with the lysosome to form an *AUTOLYSOSOME*.
The lysosome is filled with various types of enzymes (proteases, hydrolyses, etc.) capable of degrading different substance. With the formation of the autolysosome, degradation of the internal debris occurs. Lastly, the resulting amino acids and other small particles are recycled into the cell and are used to build other structures. Failure at any step of autophagy results in failure of the entire process. |
|
|
|
What is Apoptosis?
What is it characterized by? Does it result in inflammation? |

Apoptosis is actually a very late process! When apoptosis occurs, the cell has already been coping with the cellular insult for as long as 50 years! The cell simple cannot deal with whatever the cellular insult is thru autophagy or via the proteosome, and apoptosis becomes activated.
|
In most cases, apoptosis does not result in inflammation of the surrounding tissue, however it does have inflammation in the brain because surrounding microglia (mostly) and astrocytes are all activated to dispose of all the apoptotic cells and debris.
These cells stay in their current location, causing inflammation. Immune cells in the rest of the body can clear debris and return to circulation, thus avoiding inflammation. |
|
|
Apoptosis and Caspases
|
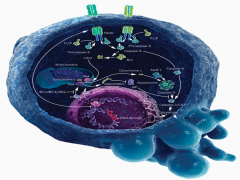
Apoptosis is mediated through caspase protein activation.
In acute neurologic diseases (e.g., stroke, traumatic injury), both necrosis and caspase-mediated apoptotic cell death occur. By contrast, in chronic neurodegenerative diseases, caspase activation has the dominant role in mediating cell dysfunction and cell death. |
|
|
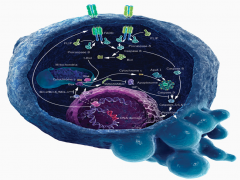
Explain the 2 pathways that mediate Apoptosis:
-Extracellular Pathway -Mitochondrial Pathway |
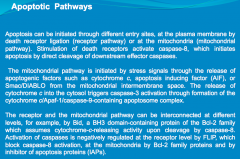
Extracellular: CD95 and TRAIL, known as the "death receptors" respond to stimuli on the cell surface. They both activate Caspase 8. From here 1 of 2 pathways can occur:
1. Caspase 8 may activate the mitochondria to release Caspase 9. Caspase 9, in turn, will activate Caspase 3, resulting in DNA fragmentation. 2. Caspase 8 may directly activate Caspase 3, resulting in DNA fragmentation. |
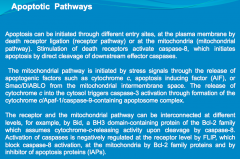
Mitochondrial: Cellular insult results in impairment of the mitochondria. The mitochondrion respond by releasing Caspase 9. Caspase 9 activates Caspase 3, resulting in DNA fragmentation.
|
|
|
What is the relationship between neurodegenerative diseases and cell death mechanisms?
|
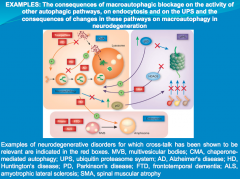
In neurodegeneration, cell death is associated with accumulation of proteins that become toxic and impair cellular quality control mechanisms, leading to apoptosis.
|
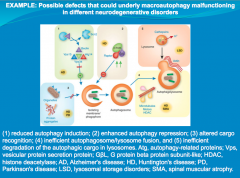
|
|
|
COMPARE forms of LTP and LTD
|
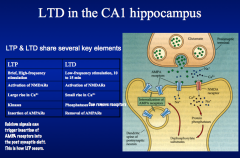
Interestingly, there is a complementary nature between LTD and LTP. LTD depression can erase an increase in EPSP caused by LTP and *vice versa*, suggesting that *LTP and LTD act at a common site to affect synaptic efficiency!*
Both LTP and LTD require activation of NMDA receptors, which allow entry of Ca+ ions into the postsynaptic cell. -Whether a synapse undergoes LTP or LTD is determined by the amount of Ca+ increase in the postsynaptic cell. |
|
|
|
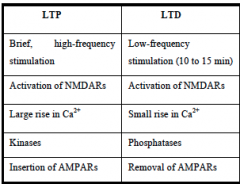
DEFINE forms of LTP and LTD
|
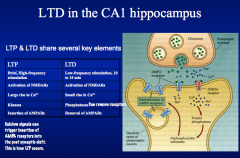
LTP requires brief high-freq stimulation.
|
LTD refers to the weakening of nerve synapses that lasts for a period of hours to days. LTD occurs at Schaffer collateral-CA1 synapses and requires low-freq stimulation for relatively long periods (10-15 min)
|
|
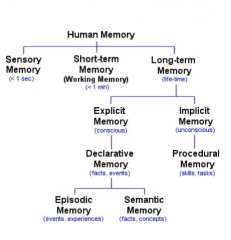
STATE the anatomical structures of the mammalian brain where explicit memory occurs.
|
COME BACK
|
|
|
|
DEFINE the Hebbian rule and how it relates to "specificity" and the associative aspect of LTP.
|

|
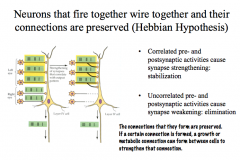
LTP formation is specific- meaning that even if LTP is induced at one synapse on a cell, it will not occur in any other inactive synapses that contact the same neuron. It is specific to the activated synapse.
LTP formation is associative- meaning that if one pathway is weakly active while a neighboring pathway on the same cell is simultaneously strongly active, then both synaptic pathways will undergo LTP. |
|
|
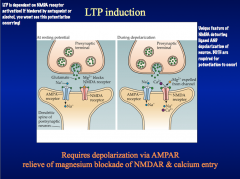
DISCUSS the role of the NMDA glutamate receptor in LTP "induction"
|
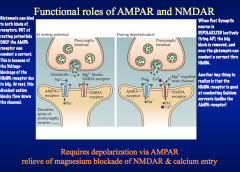
NMDA receptor channels are permeable to Ca+². When stimulated, these channels stay open 10x longer than AMPA receptors, allowing so much Ca+² to tush in that it would evoke LTP changes.
But normally, Mg+² blocks these pores. This Mg block is voltage dependent, and we've still got AMPA receptors that are receptive to lower-level synaptic activation.These mechanisms allow for the plasticity seen in LTP formation.*BLOCKING NMDA RECEPTORS WITH AN ANTAGONIST WOULD PREVENT LTP FORMATION, BUT STILL ALLOW BASIC RESPONSES TO LOW-LEVEL SYNAPTIC TRANSMISSION* |
|
|
|
What is Synaptogenesis?
-Describe the release (and staging) of the molecules involved |
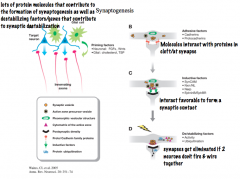
Synaptogenesis is the formation of new synapses. It requires the careful release and orientation of specific molecules
First, Netrins and Semaphorins guide extending axons to target cells, where they encounter priming factors from target neurons and glial cells. -these PRIMING FACTORS promote the maturation of axons and dendrites and prepare them for synapse formation. -Priming factors include FGF and Wnts (neuronal) and TSP (glial). Next, ADHESIVE FACTORS, including cadherins and protocadherins, promote the stabilization of the initial interaction between the axon and a dendrite or cell body Various CELL ADHESION MOLECULES (CAMs), including SynCAM and Neurorexin/Neuroligand, also promote stabilization and serve as inductive factors to promote the formation of presynaptic active zones, where NTs will be released. *Synaptic activity is the main determinant of whether or not the synapses will be stabilized or eliminated.* |
|
|
|
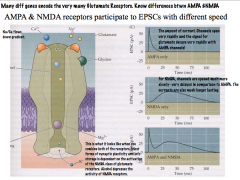
Compare how AMPA and NMDA receptors participate in EPSCs?
|
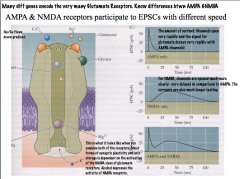
|
|
|
|
What are the functional roles of AMPAR and NMDAR?
|
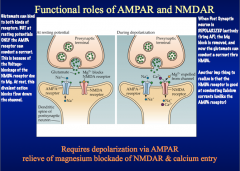
|
|
|
|
NMDA Receptor Subunits
|
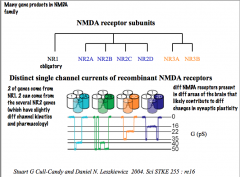
|
|
|
|
Is information storage correlated with change in synaptic efficacy?
|
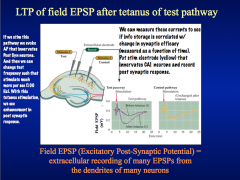
|
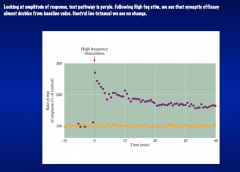
|
|
|
Explain the induction and properties of LTP
|
|
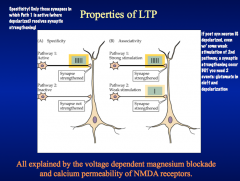
|
|
|
How does LTD work?
|
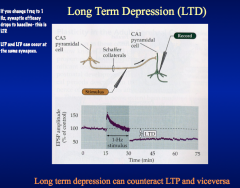
LTD requires the activation of calcium-dependent pho sphatases. And it requires the LOSS of AMPA receptors.
|
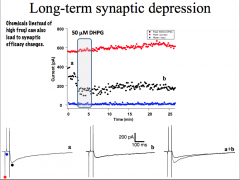
|
|
|
How can indirect channel gating by "slow" NTs lead to the production of "slow EPSPs"?
|
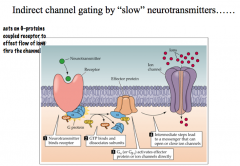
|
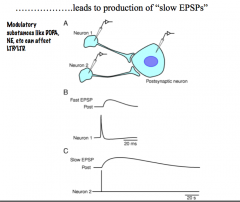
|
|
|
What is plasticity in the developing brain?
|
|
|
|
|
What is the critical period of CNS development?
|

Our brains are most susceptible to changes during a specific window early in life deemed the CRITICAL PERIOD. The critical period is the restricted developmental period during which the brain is particularly sensitive to specific experiences and environmental factors.
-this is the time where a particular experience must occur for associated behaviors to develop normally. -once this critical period ends, our brains are largely unaffected by additional experiences; failure to be exposed to the necessary stimuli during the critical period may lead to behavioral deficits that are impossible to correct. |
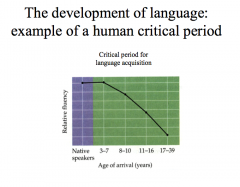
|
|
|
What are cognitive enhancers?
|
|
|
|
|
Inflammation and Pro-Inflammatory Mediators
|
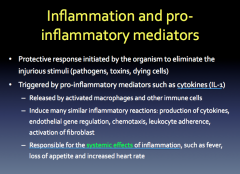
|
|
|

What causes it?
|

|
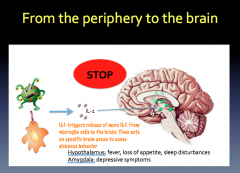
|
|
|
How does the brain regulate the immune response?
|
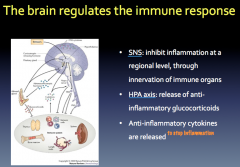
|
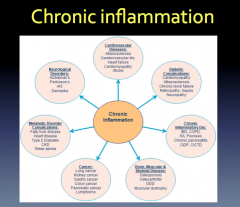
|
|
|
|
|
|
|
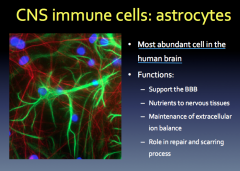
CNS Immune Cells: Astrocytes
|
|

|
|
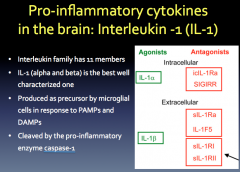
Role? Production? Relation to Neurodegeneration?
|
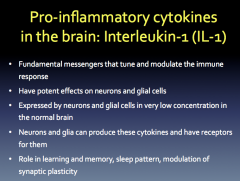
|
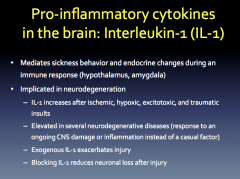
|
|
|
MS Symptoms
|
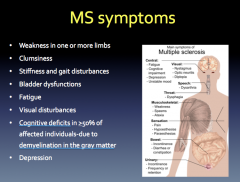
|

|
|
|
MS Etiology
|
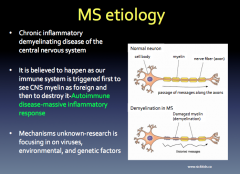
|
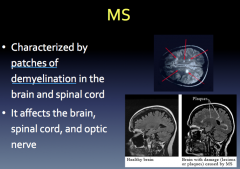
|
|
|
MS: Inflammation and Demyelination
|

|
|
|
|
MS: Neurodegeneration
|

|
|
|
|
Gray Matter Degeneration in MS
|
|
|
|
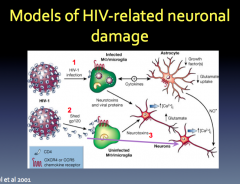
How does HIV enter the CNS?
|

|
|
|
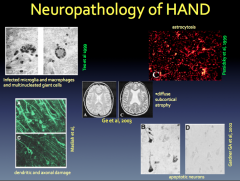
HAND Classification and Clinical Manifestation
|
|

|
|

|
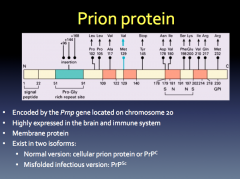
Prion Protein Functions:
-required for peripheral myelin maintenance -involved in synaptic plasticity in the neonatal hippocampus |
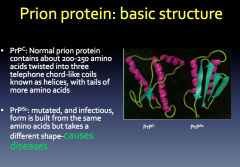
|
|
|
CJB Symptoms and Etiology
|

|
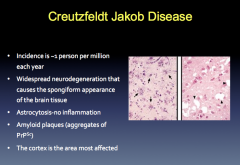
|
|
|
Prion Propagation
|
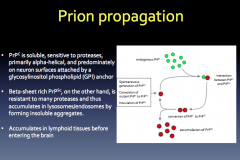
|
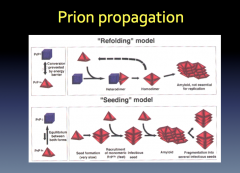
|
|
|
Fatal Familial Insomnia
|

|

|
|
|
How are Prions different from Bacteria & Viruses?
|

|

|
|
|
What are the components of the forebrain?
|

|
|
|
|
What are the 3 types of Cortex in the Cerebrum?
|
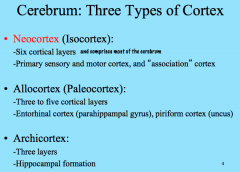
|
|
|
|
Neocortex
|

The neocortex is relatively thick and highly folded to increase surface area and the number of columns (computational units).
It completely surrounds the cerebrum to form the superficial layer of the brain. The neocortex has 6 layers in distinct columns and different regions have different types of cells and connections. Functions vary by cytoarchitectonic region. |
|
|
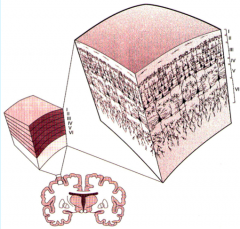
Describe the 6 Layers of the Neocortex
|

Key Points:
-Layer I has no cell bodies, receives apical dendrites from Layers III, V, and VI -Layer II has local ipsilateral projections -Layer III can project ipsilateral or contralateral to other cortical regions -Layer IV receives projections FROM the thalamus; Layer VI projects TO the thalamus -Layer V is corticofugal, projections to non-cortical regions EXCEPT THE THALAMUS |
|
|
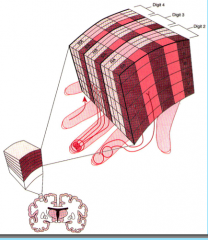
Columnar Organization of the Neocortex
|

The 6 layers of the neocortex are also organized into columns that run through the layers.
A column is formed from locally interconnected neurons running through all 6 layers to form the basic processing modules of the brain. Columns are formed from Radial Migration during cortical development. |
|
|
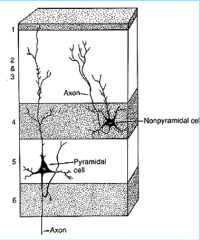
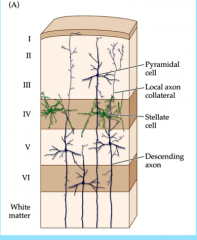
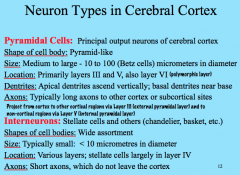
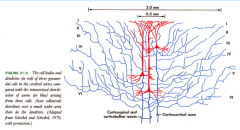
Neuron Types in Cerebral Cortex
|
|
|
|

Brodmann's Area 4
|
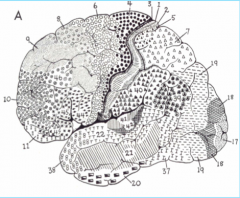
Primary Motor Cortex, in the pre central gyrus (frontal lobe)
|
|
|

Brodmann's Area 17
|
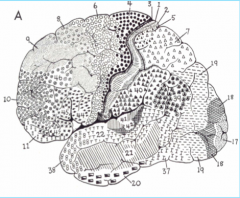
Primary Visual Cortex (occipital lone), sits mostly medially in the Calceran fissure
|
|
|
|
Brodmann's Area 41
|
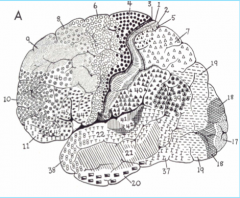
Primary Auditory Cortex, in Heschl's gyrus (above the temporal lobe)
|
|
|
|
Brodmann's Areas 1, 2, 3
|
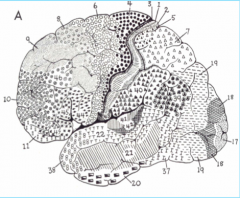
Primary Somatosensory Cortex (parietal lobe)
|
|
|
|
Brodmann's Area 6
|
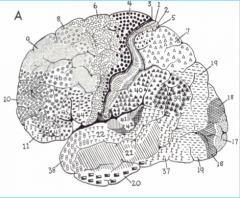
Premotor Cortex
|
|
|
|
Brodmann's Areas 44, 45
|
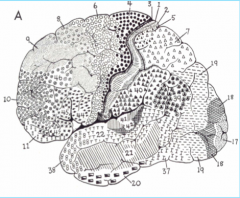
Broca's Area
|
|
|
|
Brodmann's Area 22
|
Posterior part is Wernicke's area
|
|
|
|
Brodmann's Area 39
|
Angular Gyrus
|
|
|
|
Brodmann's Area 40
|
Supra Marginal Gyrus
|
|
|
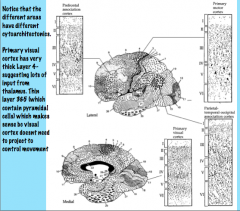
|
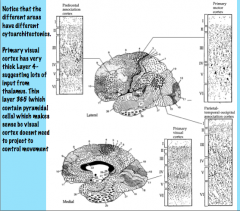
Layers III and V of the primary motor cortex (Brodmann 4) are very thick with lots of pyramidal cells because they need to project to areas such as the spinal cord and pons to allow movement.
In contrast, primary visual cortex (Brodmann 17) has a very thick Layer IV and thinner Layers III and V (less output because it doesn't control anything). Visual stimulation from the retina projects to the lateral geniculate nucleus/body of the thalamus, which projects to Layer IV of the primary visual cortex. Higher level areas have much more evenly distributed layers. |
|
|
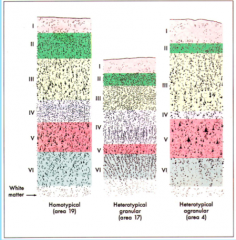
Homotypic vs. Heterotypic Cortex
|
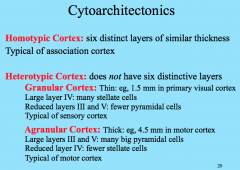
|
|
|
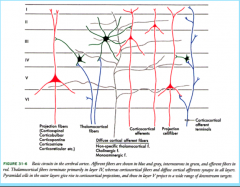
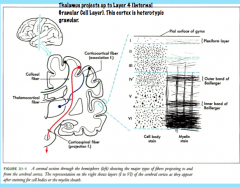
Afferent Connectivity in the Cortex
|
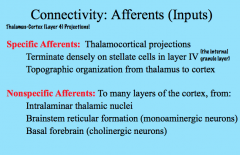
The thalamocortical (thalamus to cortex) projections project densely on stellate cells in Layer IV (internal granule layer) topographically.
|
|
|
|
Topographical Organization of the Thalamus
-Lateral Geniculate Nucleus/Body -Medial Geniculate Nucleus |
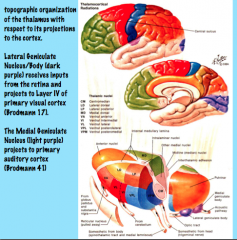
|
|
|
|
Efferent Connectivity in the Cortex
|
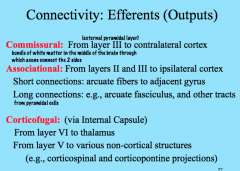
Commissural projections move contra laterally from one hemisphere to the other via commissures, which are bundles of white matter that connect the two hemispheres. They originate primarily from Layer III (external pyramidal layer).
|
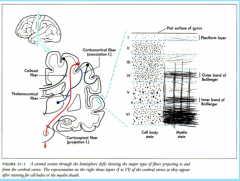
|
|
Internal Structure
-What is it? -Where is it? -What is its structure? |

|
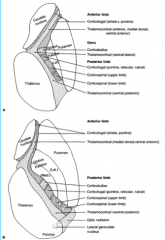
|
|
Commisures
|
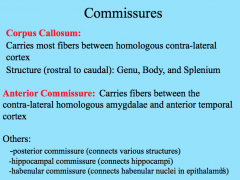
|
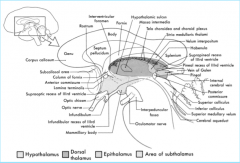
|
|
|
|
|
|
|
Lesion Studies
-What is it? -Pros? -Cons? |

This method correlates loss of function with areas of damage, or lesions. Focal lesions occur in those with traumatic brain injury or strokes, and widespread lesions occur in those with neurodegenerative disorders.
PRO: you can identify specific function areas CON: lesions are not always "clean", or specific to one area. Also, plasticity and compensation come into play and you may wrongly attribute a function to a part of the brain that has simply taken over for the damaged structure. |
|
|
|
Functional Neuroimaging: PET, fMRI
-Method? -Pros? -Cons? |
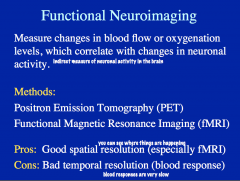
This method identifies changes in blood flow or oxygenation in brain structures, which are associated with neural activity.
|
|
|
|
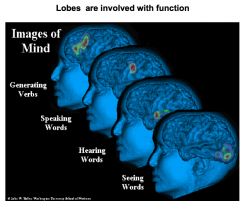
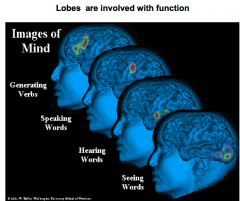
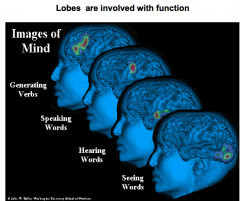
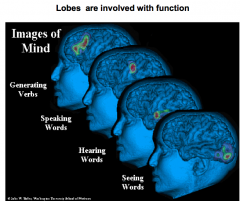
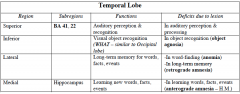
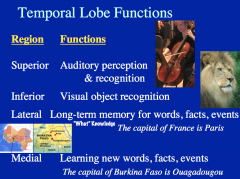
Name the 4 main function brain regions
|
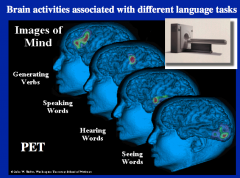
generating verbs = prefrontal lobe
speaking = Broca's hearing = temporal lobe seeing = occipital lobe |
|
|
|
Electrophysiology: ERPs
|
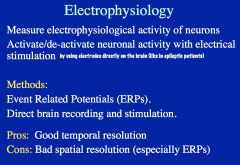
ERP method directly measures electrical activity in the brain (via EEGs), in response to a stimulus.
Direct brain recording and stimulation allows you to activate/deactivate neuronal activity by putting electrodes inside the brain itself. -this is often done to determine the source of epileptic seizures. |

|
|
|
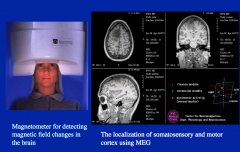
Magnetoencephalography: MEG
|

MEG measures the magnetic fields associated with electrical activity.
It is similar to EEG in that it is scalp-recorded (and therefore has the same pros and cons) |
|
|
|
Transcranial Magnetic Stimulation: TMS
|
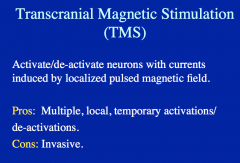
TMS allows you to activate/deactivate neurons via a pulsing magnetic field, initiating or suppressing a particular function/activity.
|
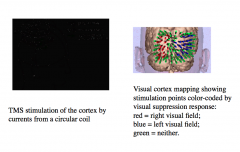
|
|

|
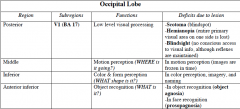
|
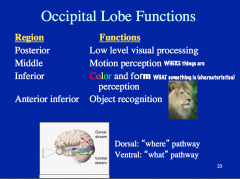
|
|

|
|
|
|
|
|
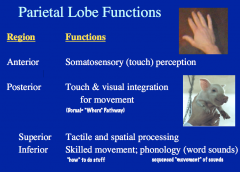
|
|

|
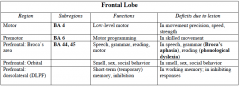
|
|
|
|
Touch and Proprioception Fibers
|

Have specialized mechanoreceptors at their nerve endings.
These fibers are unimodal, heavily myelinated, and have a large diameter. Due to their large diameter and heavy myelination, they have high conduction velocity (remember, conductance increases as you increase the diameter and myelination of an axon) |
|
|
|
Pain (Nociception) & Temperature Fibers
|
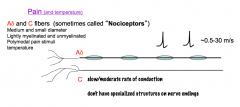
Have bare nerve endings.
The Aδ fibers are polymodal, lightly myelinated, and have a medium diameter. Due to their medium diameter and partial myelination, Aδ fibers have a medium conductance velocity. The C-fibers are polymodal, completely unmyelinated, and have a small diameter. Due to their small diameter and lack of myelination, C-fibers have a slow conductance velocity. -C-fibers are also the most abundant sensory fibers in the body |
|
|
|
Touch: Pathway through the spinal cord to the brain
|
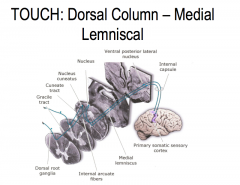
The sensory receptors of primary touch neurons are found in the periphery.
From the receptor, touch neurons travel through the dorsal root ganglion, where the cell bodies of these neurons are located, into the spinal cord. The primary touch neurons then ascend ipsilaterally through the dorsal column of the medial lemniscus until it synapses at the cuneate nucleus of the medulla. The secondary afferent neuron crosses over the midline and then ascends contra laterally to the ventral posterior lateral (VPL) nucleus of the thalamus. The tertiary touch neurons then ascends ipsilaterally to the primary somatosensory cortex. |
|
|
|
Pain and Temperature: Pathway through the spinal cord to the brain
|
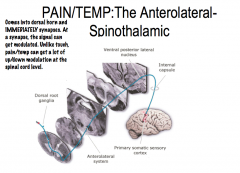
The sensory receptors of primary pain neurons are found in the periphery. From the receptor, pain neurons travel through the dorsal root ganglion, where the cell bodies of these neurons are located, into the spinal cord, where they immediately ipsilaterally synapse in the dorsal horn.
Secondary afferent neurons then cross over and ascend contralaterally via the anterolateral system to the VPL of the thalamus. The tertiary afferent neuron then ascends ipsilaterally to the primary somatosensory cortex. |
|
|
|
Trigeminal System
|
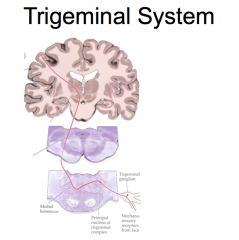
(EXCEPTION!)
The sensory receptors of sensory trigeminal system neurons are found in the face. From the receptor, sensory trigeminal system neurons travel through the trigeminal ganglion, where the cell bodies of these neurons are located, into the brainstem. Both primary pain and touch neurons synapse ipsilaterally immediately upon entering the brainstem. The secondary afferent then crosses over, and ascends contralaterally to the thalamus. The tertiary afferent neuron then ascends ipsilaterally to the primary somatosensory cortex. |
|
|
|
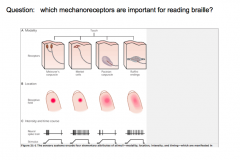
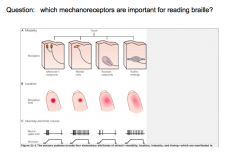
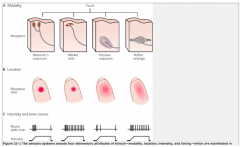
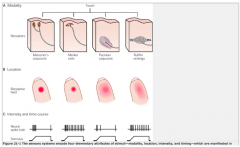
The 4 Types of Mechanoreceptors
|
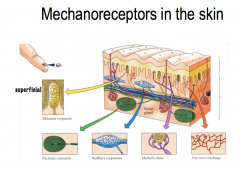
Touch and pressure are transducer by specialized mechanoreceptors at the peripheral terminus of touch neurons (Aα and Aβ fibers). THey have different receptive fields (the area of skin that nerve responds to).
|
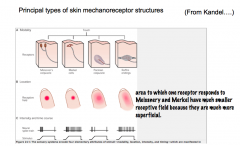
Meissner's Corpuscle and Merkel Disks are more superficial (located closer to the skin. Therefore, these 2 have smaller receptive fields than the deeper Pacinian and Ruffini's Corpuscles.
They also differ in their ability to adapt to constant pressure. -Meissner's and Pacinian Corpuscles rapidly adapt to constant pressure; thus, they fire when the pressure changes, but stop signaling during constant pressure. -Merkel Disks and Ruffini's Corpuscles do not adapt to constant pressure, and thus continue to signal during constant pressure. |
|
|
Meissner's Corpuscles and Merkel Disks.
|
|
|
What mechanoreceptors detect sand paper roughness?
|
Meissner's Corpuscles and Merkel Disks
|
|
|
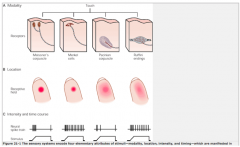
What mechanoreceptors register a pen tapped on your finger?
|
All!
The superficial mechanoreceptors would be activated by the contact of the pen against the skin, and the deeper mechanoreceptors would be activated by the vibration of bone caused by the tapping. |
|
|
|
Mechanoreceptor Density
|
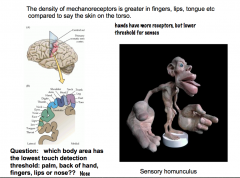
The density of mechanoreceptors varies in different parts of our body.
Our hands and areas of the face are very sensitive to touch, because they have a greater density of mechanoreceptors. Because of the greater density in these areas, there is a greater amount of sensory cortex in the brain associated with these regions. The sensory homunculus is a visual depiction that shows this characteristic. For example, the hands have a much larger area of the sensory cortex associated with them than the legs, even though our legs are much larger than our hands |
|
|
|
Mechanosensation- How are touch neurons able to transduce a mechanical stimuli into a signal?
|
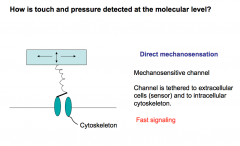
Direct Mechanosensation:
There are mechanosensitive ion channels that are tethered to the extracellular matrix and intracellular cytoskeleton. Upon deformation of the extracellular matrix, the channel is opened leading to depolarization of the touch neuron. *Fast touch signaling is attributed to Direct mechanosensation* |
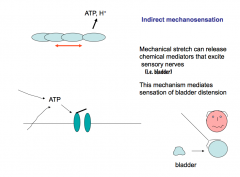
Indirect Mechanosensation:
Mechanical stretch can release chemical mediators that excite sensory nerves. If you stress epithelial cells, they release ATP, which then activates nearby nerves through purinergic signaling. This mechanism mediates sensation of bladder distention. |
|
|
Explain how Pain Molecules mediate pain and sensory nerves
|
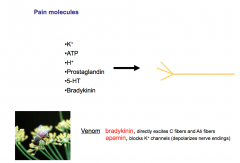
If you rupture a cell or cause trauma to tissue- ATP and Potassium, which are relatively abundant intracellularly, will be released from the cell. ATP through purigenic signaling can lead to stimulation of nociceptive neurons.
Raising extracellular potassium leads to increased depolarization of nerves, including pain nerves. Protons are released during ischemia and are an important stimulator of intense ischemic pain. Arachidonic Acid forms prostaglandins, which are key mediators of inflammation and pain. Serotonin is found in the gut and platelets, and is released due to cuts or gut injuries. Serotonin is a potent pain molecule. Bradykinin circulates as a precursor through the blood and is activated by trauma and is a potent pain activator. Bee venom is packed with bradykinin (directly excites C-fibers and Aδ fibers) and apamin (blocks K+ channels, which depolarizes nerve endings). |
|
|
|
Pain Receptors
|
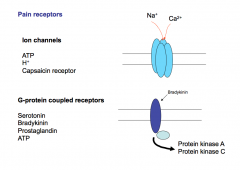
There are 2 types of pain receptors- ion channels and G-protein coupled receptors. Every pain molecule can act on BOTH kinds of receptors.
Ion channels are thought to be faster and to mediate faster responses. GPCRs are thought to be modulatory and cause up or down regulation of pain reception, but this is not always the case. Pain GPCR activation typically leads to phosphorylation of pain ion channels, making it much more sensitive to opening. |
|
|
|
Capsaicin Receptors
|
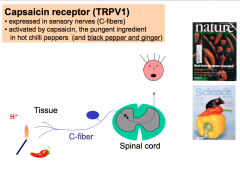
The capsaicin receptor is expressed in 50-60% of C-fibers. This receptor normally responds to an Endogenous Lipid (which capsaicin, the pungent component in chili peppers mimics), Acid Stimuli, and Thermal Stimuli (temperatures at or above 43 degrees Celsius).
The capsaicin receptor is activated by and integrates multiple inflammatory inputs (an "inflammatory soup"). This receptor is important in inflammatory pain and hyperalgesia. When activated, this receptor causes long-lasting, dull, burning pain. -Mice that have the capsaicin receptor knocked out have no inflammatory chemical or thermal pain. |
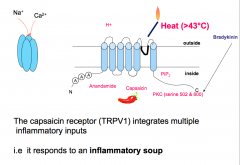
|
|
|
Mustard Oli Receptor (TRPA1)
|
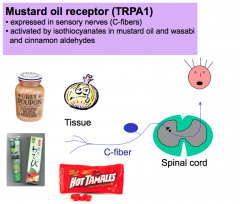
The mustard oil receptor is also expressed in C-fibers. This receptor responds to chemicals, isothiocyanates, found in mustard oil and wasabi and cinnamon aldehydes.
|
|
|
|
Understand the concept of "primary pain" (acute response) and "secondary pain" (hyperalgesia)
|
Aδ fibers mediate PRIMARY PAIN due to their ability to conduct APs significantly faster than C-fibers. Primary pain is a rapid pain response to an acute noxious insult, like touching a hot stove. The acute response helps to prevent danger and avoid injury; like the instinctive recoil reflex away from the noxious stimuli that minimizes thermal damage to our tissue.
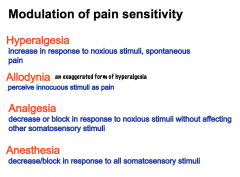
C-fibers normally DO NOT conduct a pain response unless there is inflammation or damage. Injured tissue is tender, because C-fibers become active. C-fibers fire at a slow rate and we experience this as a dull, burning, long-lasting pain. This PROLONGED HYPERSENSITIVITY facilitates us to stop using the tissue so it can repair itself. |
Prolonged hypersensitivity is also referred to as HYPERSENSITIVITY (an increase in response to noxious stimuli).
Allodynia is a form of hyperalgesia in which previously innocuous stimuli are perceived as pain. An example of this is when you have a sunburn and someone slaps you on the back. Previous to the sunburn, a slap on the back is not a noxious stimulus. Analgesia is the decrease or block in response to noxious stimuli without affecting other somatosensory stimuli. Anesthesia is the decrease or block in response to all somatosensory stimuli. |
|
|
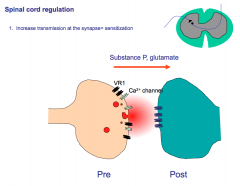
Describe how pain signaling is up- or down-regulated in the spinal cord and appreciate the integration between touch and pain transmission.
|
Depending on one's subjective and emotional state, one's perception of pain is different.
-if a person is stressed/depressed, they experience more pain than if they're happy. The amygdala and other non-primary somatosensory cortex regions create the emotional state of pain. This is much different than touch, which is NOT impacted by someone's subjective state. When pain fibers come into the spinal cord they immediately synapse ipsilaterally at the dorsal horn. At this synapse you can get modulation of the signal. Bursts of APs from Aδ or C-fibers will cause facilitation/sensitization of the synapse, which is similar to long term potentiation. This leads to enhanced transmission of pain that can last for several days. Opioids block this synapse well. |
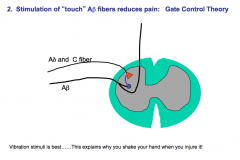
Stimulation of Aβ fibers can send off collaterals that send inhibitory signals to Aδ and C-fibers that can gate out and diminish the pain signal.-This theory is known as gate control theory.
Touch sensation also reaches the brain before pain sensation due to faster conduction along the Aβ fibers. The brain can then send descending inhibitory signals to the pain fibers, as well, which leads to a decrease in nociception. This is the basis of acupuncture analgesia- the use of a vibratory stimulus that maximally stimulates touch receptors. Some of the touch interneurons may be opioid interneurons. |
|
|
Describe key features of temperature receptors
|

5-10% of C-fibers are not involved in nociception, and instead function to record the ambient room temperature.
These C-fibers have TRP channels, ion channels that admit Na and Ca into the cell, which allow them to carry on their temperature sensing function. |
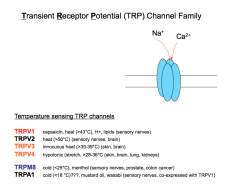
There are several subtypes of these channels that are optimally stimulated by different temperature ranges. At any given temperature, some of these subtypes are on and others are off. The INTEGRATION of the signals generated by the activated subtypes allows for the sensation temperature.
Capsaicin receptors also play an important role in THERMOREGULATION. In the body, they can be sensitized to be activated at a lower temperature. When activated, they can trick a person into thinking they are hyperthermic- this leads to stimulation of a cooling response, which is why spicy food makes us sweat. |

