![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
38 Cards in this Set
- Front
- Back
|
single gene disorder |
the genetic abnormality is the sole cause of disease |
|
|
multifactorial disease |
genetic variations interact with a wide variety of other factors leading to disease |
|
|
chromosomal disease |
-abnormal # of chr. or abnormal chr. structure |
|
|
mitochondrial disease |
rare type of disorders caused by mutations in non-chromosomal mtDNA |
|
|
mutations and disease (same gene diff disease) |
-~10,000 disease attributed to mutations in ~6000 genes -more diseases attributed to mutations in single genes -diff mutation in 1 gene+ diff diseases in same gene (heterogenety)
e.g mutation in FGFR-3
An example:
Mutations in FGFR-3
P250R – Muenke’s syndrome G308R – Achondroplasia (dwarfism)
|
|
|
mutations and disease (many genes 1 disease) |
e.g Xeroderma pigmentosum (XP) ‘Children of the Night’
|
|
|
what is penetrance |
-The frequency with which a person manifests the gene that they possess
-Not all dominant mutations display 100% penetrance
-Penetrance is determined via genetic and environmental factors
|
|
|
what is expressivity |
Variation in the severity of the symptoms caused by a mutation
For example, in sickle cell anaemia (which is always caused by the same mutation) symptoms range from very severe to extremely mild
|
|
|
what's a phenocopy |
|
|
|
what is mosaicism |
– different diseases
|
|
|
other factors which affect genetic conditions |
Genomic Impriting:he expression of a gene depends of the parent from which the gene is inherited. The imprinted (inherited) allele is silenced (epigenetic: DNA methylation, histone modification), expression from non-imprinted allel |
|
|
point mutation diseases |
Substitutions (missense)
E6V Nonsense mutations
K1524X
nsertion mutations
Familial Hypercholesterolemia (FH) results in elevated levels of blood lipids. A genetic study was carried out on a large consanguineous Pakistani family with a history of FH and a common insertion mutation was identified. c.2416_2417InsG
Deletion mutations
Around 80% of cystic fibrosis patients in Western Europe harbour a deletion mutation that deletes the phenylalanine (F) residue at position 508 in the protein. ΔF508
|
|
|
What type of tests are carried out?
|
(test for parents if carry one copy of mutation, that in 2 copies causes a disorder)
|
|
|
what is a complex disease |
a on- monogenic disease e.g Cancer Asthma Migraine Diabetes (I and II) Arthritis MS Hypertension Cardiovascular disease Obesity Crohn’s disease Schizophrenia Autism Alzheimer's |
|
|
what is heritability ? |
|
|
|
what is concordance |
|
|
|
Classical studies used to investigate the genetic contribution to a disease
|
|
|
|
alleles shared between families |
1st degree 50% Parent/Child, sibling
2nd degree 25% Grandparent/Grandchild, Aunt or Uncle/Niece or Nephew
3rd degree 12.5% Cousins, great grandparent/great grandchild |
|
|
Fisher’s theory |
|
|
|
‘liability threshold’ determines disease state of an individual |
|
|
|
familial breast cancer allele |
BRCA1 |
|
|
how are susceptibility alleles identified |
using Parametric analysis A type of linkage analysis that uses LOD scores (>LOD >linkage)
|
|
|
Non-parametric analysis |
A type of linkage analysis that analyses co-inheritance
The premise is that alleles which predisposed an ancestor to a disease will be inherited by affected family members at a frequency greater than expected
|
|
|
what is transformation |
|
|
|
what triggers cell transformation ? |
oncogenes |
|
|
|
|
|
how do proto-oncogenes become activated |
|
|
|
tumour suppressors |
-supress cell transformation -negativelly reduce cell growth
- In other words, both alleles must be mutated in order for the gene to stop functioning (Knudson’s 2-hit hypothesis)
|
|
|
how many divisions do normal cells undergo before dying |
50 |
|
|
what causes cells to die |
shortening of telomeres
|
|
|
what enzyme is reactivated in cancerous cells |
telomerase |
|
|
what gene therapy |
|
|
|
Transgene delivery |
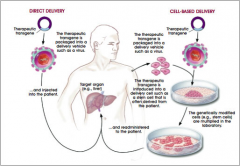
|
|
|
mostly used widely used vehicles |
virusese.g- • Adenoviruses e.g. common cold • Herpesviruses e.g. cold sores • Retroviruses e.g. HIV |
|
|
comparison of gene therapy |

|
|
|
what disorders best suit gene therapy |
single gene recessive disorder |
|
|
Adenovirus gene therapy |
Head and neck cancer
|
|
|
Retrovirus gene therapy
|
SCID-XI X-linked severe combined immunodeficiency (X-SCID)
|

