![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
18 Cards in this Set
- Front
- Back
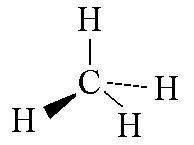
Name this shape
|
tetrahedral
|
|
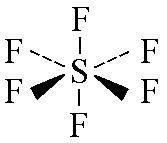
name this shape
|
Octahedral
|
|
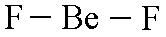
name this shape
|
linear
|
|
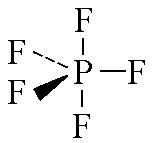
name this shape
|
trigonal biyramid
|
|
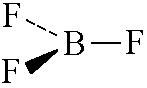
name this shape
|
trigonal planar
|
|
|
which shape has three bonded pairs and one lone pair of electrons?
|
triangular pyramid
|
|
|
which shape has 2 lone pairs and 2 bonded pairs?
|
v-shaped
|
|
|
what is the shape of a water molecule?
|
v-shaped
|
|
|
what shape does an ammonia (NH3) molecule have?
|
triangular pyramid
|
|
|
what shape is chlorine tetrafluoride ion? what must you remember to add on when drawing it?
|
square planar. brackets and the charge.
|
|
|
what is the bond angle in a v-shape molecule?
|
104.5
|
|
|
what is the bond angle in a triangular pyramid?
|
107
|
|
|
what is the bond angle of a trigonal planar?
|
120
|
|
|
what is the bond angle in a tetrahedral?
|
109.5
|
|
|
what is the bond angle in a trigonal bipyramid?
|
120
|
|
|
what is the bond angle in a octahedral?
|
90
|
|
|
what repels more a lone pair or a double bond?
|
double bond
|
|
|
what is ethene (C2H4) shape? what are the bond angles?
|
planar- 121 and 118
|

