![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
37 Cards in this Set
- Front
- Back
|
Intracellular motility and intercellular spread |
.. |
|
|
to continue to replicate |
-intracellular pathogens must move from one host cell to another
- for most pathogens bacteria replicate in a membrane bound compartment until the host cell lyses and newly created bacteria then repeat their invasion process into a new cell
-small number of bacteria have developed mechanisms for intracellular and intercellular spread prior to lysis
- these bacteria leave the phagosome by secreting membrane degrading enzymes to reach the cytosol |
|
|
to continue to replicate |
- the cytosolic bacteria utilize the host actin cytoskeleton to move within the cell and to spread to a neighboring cell. this allows bacteria to move from one cell to the next without being exposed to antibodies or innate IS components
-bacteria known to do this are : listeria, shigella, rickettsia, mycobacterium, and burkholderia. |
|
|
targeting the Arp2/3 complex |
- each of these motile pathogens promotes actin polymerization by exploiting a host protein complex called the arp 2/3 complex, which is conserved molecular machine used by host cells to nucleate actin filaments and organize them into branched arrays.
-each bacterial pathogen utilizes the host arp2/3 it appears the have adapted different strategies for interacting with and activating the arp2/3 complex showing that they have independently evolved the capacity to undergo actin base motility |
|
|
actin filament assembly by host cells |
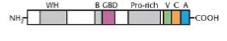
- to initiate motility pathogens first stimulate the nucleation of actin filament assembly at the bacterial surface by making actin nucleation promoting factors that in turn activate arp2/3 complex
-in host cells the arp2/3 complex is activated by a class of cellular proteins called nucleation promoting factors NPFs
-most NPFs (WASP) contain several conserved domains including proline-rich region, C-terminal WASP homology 2 region (V in figure below) that binds actin monomers and c-terminal central and acidic regions (c and a) that bind to the arp2/3 complex (b and gbd bind to PIP2 and Cdc422)
|
|
|
nucleation of actin polymerization by pathogens |
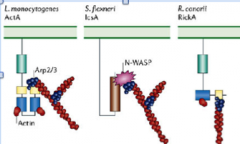
intracellular pathogens have adopted two main strategies for activating the arp2/3 complex:
1.) either they express mimics of WASP family members (listeria monocytogens ActA or rickettsia conorii RickA)
2.) they express surface proteins that recruit host wasp proteins (shigella flexneri IcsA recruits N-WASP)
|
|
|
nucleation of actin polymerization by pathogens |
- once actin filaments are nucleated and organized into Y -branched networks by the arp 2/3 complex at the cell surface of the bacteria cell, filament elongation occurs, generating a force that drives motility
- elongation is eventually terminated at the ends of older filaments by capping protein CapZ, leaving only the end of newly nucleated filaments at the pathogen surface free to polymerize
-as filaments age and hydrolyze ATP older ADP containing filaments in the array are severed and depolymerized (by cofilin etc) |
|
|
mechanisms for promoting actin nucleation for each pathogen involve different strategies
A.) listeria |
- listeria invades epithelial cells then secretes a pore forming hemolysin called listeriolysin O (LLO) which degrades the phagosome
-upon entering the cytosol teh bacteria are surrounded by a cloud of short actin filaments which subsequently localize to on end of each bacterium after cell division
- the actin rich tail forms and the bacteria begins moving in the cytosol at speeds ranging from 6-90 mico meters/min |
|
|
listeria cell-to-cell movement |
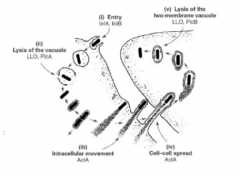
|
|
|
mechanisms for promoting actin nucleation for each pathogen involve different strategies
A.) listeria |
- the actin tail can be tens of microns long and is made up of many thousands of short actin filaments cross linked into a dense mesh work
- the actin filaments are all organized with the growing plus end oriented against the bacterium, while the tail actin filaments remain stationary within the cytoplasm
- the filaments are growing at teh bacterial surface, thus the propulsive force is derived by the actin polymerizing at the bacteria cell wall
-plus end at bacteria surface pushes actin forward |
|
|
ActA from listeria |
- one bacterial protein, ActA is necessary and sufficient for listeria motility. expression of ActA in other bacteria such as strep, results in those bacteria becoming motile inside mammalian cells
- ActA (67kda) is expressed uniformly on bact. cell surface until the bacteria divides at which time ActA becomes polarized to one end, there by accounting for the polarized actin tail formation
-actA catalyses the nucleation and polymerization of actin filaments on the bact. surface and it catalyses the rearrangements of these filaments into a polarized tail structure that causes unidirectional motion
-finally actA is important for the speed of movement |
|
|
ActA structure |
- ActA is not homologous to any proteins
- it has an N signal seq and a C membrane spanning domain
- the N one-third of the protein mediates ActA dimerization and is also necessary for actin filament nucleation
- the central one-third of the ActA contains four proline rich repeats that affect the rate of moving. removal of each repeat one at a time reduces movement speed by 20%
-ActA has none of these effects on bact. in vitro. all of these activities depend on ActA interacting with several host cellular factors inside living host cells |
|
|
ActA structure |
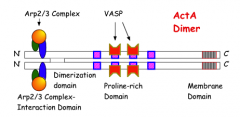
|
|
|
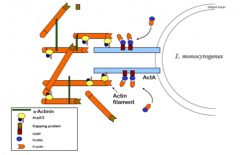
host factors |
-several host factors interact directly or closely with actA including the arp2/3 complex (which has 7 proteins), profilin, VASP, and alpha actin
|
|
|
host factors
- arp 2/3 complex |
has actin nucleating activity by itself but acts synergistically for nucleating activity with actA
- arp2/3 interacts with actA N one-third to inititate actin nucleation at the growing end of the actin tail that is pushing the bacterium
-VASP modulates this actibity by interacting with the long proline rich repeats in teh actA central domain |
|
|
host factors
-VASP |
binds directly to actA at the consensus seq FPPPP found in each of the four proline rich repeats
-deletion of proline sequences abolishes VASP binding
-after binding actA, VASP binds profilin, linking profilin to actA |
|
|
host factors -profilin |
binds actin monomers and stimulates release of ADP and binding of ATP by G-actin
-profilin also acts like a chaperone to deliver G-actin::ATP to the growing end of the actin tail at the bacterial surface
-profilin does not bind actA directly. profiin must bind VASP which in turn binds ActA
-the decrease in actin polymerization on the growing actin filament caused by deleting proline repeats results in a decrease in the speed of bacterium , both of which are due to reduced VASP binding and hence reduced profilin binding
-reduced profilin binding will slow the delievery of G-actin::ATP |
|
|
host factors -alpha actin |
is an actin cross-linking protein that forms homodimers that associate with an actin filament at each end and can cross link actin filaments in the actin tail.
this cross linking function is required primarily for establishing the mechanical integrity of the tail that enables it to push the bacterium |
|
|
alpha- actin |
|
|
|
host factors B.) shigella |
- shigella flexneri enters cells by a completly distinct mechanism from listeria and exists in a ery diff vacuole. like listeria it quickly excapes the vacuole in this case by an IpaB-dependent mechanism
-once inside the host cytoplasm, shigella lifestyle is similar to that of listeria. shigella also forms an actin tail that is used for motility.
-shigella motility depends on the IcsA protein which has no homology with ActA from listeria E.coli that are made to express IcsA become motile therefore IcsA is the only bacterial protein necessary for bacterial motility
-like for listeria many host proteins are required for the IcsA- based motility of shigella |
|
|
Shigella -IcsA localization |
-IcsA is a protein with an N region called the alpha domain which is exracellular and orientaed away from the cell
-the IcsA is delivered to one pole of the bacterium but slowly diffuses around the bacterium creating a gradient
-a membrane bound bacterial protease cleaves the IcsA and helps maintain the gradient. thus IcsA is polarized to on end of shigella just as ActA is polarized to one end of listeria |
|
|
shigella -actin filament assembly by IcsA |
-host proteins required for shigella motility: WASP, vinculin, arp2/3, VASP, profilin, and alpha-actin
-shigella's IcsA does not bind directly to arp2/3 complex. it does bind to N-WASP which then binds and activates the arp2/3 complex which mediates actin polymerization
-IcsA also binds binculin but this binding is not essential for motility rather it controls the speed of shigella
-this is because vinculin also binds to VASP which in turn binds profilin and thus increases the concentration of G-actin::ATP available to rapidly elongate the actin filaments |
|
|
shigella -how important is intracelluar spread in the disease caused by shigella |
a shigella mutant with a null icsA allele (makes no IcsA protein) is unable to spread from cell to cell and causes only a small foci of infection overlying Peyer's patches (where M cells are) in a simian model of infection. Thus the mutant lacking icsA is almost non virulent |
|
|
the shigella and listeria motility stystem comparisons |
1.) one bacerial surface protein (listeria: ActA, shigella: IcsA) binds to host factors to cause actin filament nucleation listeria: ActA --> Arp2/3 --> F-actin shigella: IcsA --> N-WASP --> arp2/3 --> F actin
2.) for both shigella and listeria nucleated actin forms a polarized tail, cross-linked by alpha actininin to generate unidirectional movement
3.) actin filament elongation at teh bacterial surface is accelerated by host factors listeria: ActA-->VASP-->profilin-->G-actin-->ATP Shigella: IcsA->vinculin->VASP->profilin->G-actin-->ATP
4.) the dynamics and behavior of the actin tail are controlled solely by host factors and normal homestatic mechanisms
5.) both shigella and listeria move using only host energy |
|
|
host factors C.) rickettsia |
-rickettsia species fall into two groups the spotted fever group and the typhus group
-R. conorii: the agent of the mediterranean spotted fever and R. rickettsii, the agent of rocky mountain spotted fever exhibit actin-based motility in teh cytosol of host cells
-the velocity of motility is about 5-8 micro meters/min which is about half the rate of L monocytogens and S. flexneri
-rickettsia exhibit some striking differences from listeria and shigella the most pronounced of which relates to the organization of actin filaments in their comet tails |
|
|
host factors C.) rickettsia |
-R. rickettsii tails comprise tow or more distinct bundles that wrap around each other in a helical fashion
- rickettsia tails comprise long filaments arranged in nearly parallel arrays, distinct from the branched organization seen in tails formed by listerial monocytogens |
|
|
RickA |
R conorii has the Rick A gene which encodes a protein similar to the human WASP family of NPFs
-rickA is expressed on the bacterial surface and behaves like L.monocytogens ActA and other NPFs in its ability to activated the arp2/3 complex in vitro |
|
|
arp2/3 role in rickettsii |
- the arp2/3 complex is detectable around intracellular bacteria before tail formation
-consistent with the long unbranched filament architecture, arp2/3 complex is not detected along the length of rickettsia tails by immunofluorescence microscopy
-apparently arp2/3 is used only transiently to initiate actin polymerization on a bacterial surface
-once polymerization is initiated the arp2/3 complex is not necessary for actin filament elongation and sustained motility and is removed
rickA + arp2/3 --> rickA::arp2/3 + g-actin --> rickA -->arp2/2 --> f-actin |
|
|
D.) mycobacterium |
- M. marinum causes a systemic tuberculosis like disease in fishes and frogs and a localized disease in humans
-until recently it was though that pathogenic mycobacteria did not excape from the phagosome into the cytosol of macrophages. however a recent report demonstrates that a fraction of M. marinum can in fact excape from the phagosome.
-once in the cytosol mycobacterium are propelled using actin based motility at rates of about 11 micro meter a min
-actin based motility may be involved in cell-to cell spread of m. marinum |
|
|
D.) mycobacterium |
- the mechanism of actin polymerization by M. marinum appears to be most similar to shigella
-m. marinum uses an unknown bacterial protein (protein X) to initiate actin polymerization by recruiting WASP. WASP is concenrated at the bacterial pole from which actin polymerizes
-similarly the arp2/3 complex is localized throughout actin tails as is VASP
protein X --> WASP --> arp2/3 --> actin protein X--> VASP--> profilin-->G-actin/ATP |
|
|
E.) burkholderia |
-burkholderia pseudomallei causes meloidosis a disease that affect humans and animals in tropical areas. infection by environmental organisms generally occurs via cutaneous inoculation. the clinical manifestation are divers and range from localized sub-acute infection to pneumonia and sepsis |
|
|
E.) burkholderia BimA |
-the molecular mechanism of actin polymerization by B. pseduomallei is largly unknown. however motility depends on BimA a polarized protein that is beleived to promote actin nucleation
-it was recently shown that arp2/3 complex is localized throughout actin tails formed by this bacterium suggesting a role for the complex in actin based motility |
|
|
intercellular spread of bacteria -typical bacterial motility is useless |
- the main utility of actin based motility is the ability of the bacteria to reach the host cell surface and spread to a neighboring cell
-bacteria cant diffuse far in viscous host cytoplasm and bacterial flagella cant rotate in the viscous environment
-the host's actin cytoskeleton provides a ready solution that can be easily exploited by bacteria merely by expressing a single protein on their surface, thereby providing binding sites for host factors that spontaneously and rapidly polymerize actin at the bacterial surface
-all subsequent functions are done by host factors |
|
|
intercellular spread of bacteria -benefits of cell to cell movement |
intercellular spread allows pathogens to move from the cytoplasm of one cell to the cytoplasm of the neighboring cell, therby avoding immune factors such as antibodies complment and phagocytic cells. unfortunately for us such movement allow pathogens to cross the placental and blood brain barriers
|
|
|
intercellular spread of bacteria mechanism of cell to cell movement |
-the process of cell to cell movement begins when a bacterium accidentally collides with the host cytoplasmic membrane. this can produce long membrane bound protruisions into adjacent cells which form at the same speed as inracellular motility and are formed by the same actin based motiltiy process |
|
|
intercellular spread of bacteria mechanism of cell to cell movement |
-the neighboring cell will pinch off the protrusion after a certain period of time
-the pinching off process appears to occur normally between epithelial cells at a low frequency and is called cytophagy. cytophagy may normally be important in tissue specification during development
-the result is the bacteria are now in a new cell but in a double membrane vacuole
-listeria escape this double membraned vacuole by expressing the same hemolysin they use durin entry by phagocytic processes, LLO plus two phospholipases (PlcA and PlcB) to disrupt the special double membrane vacuole |
|
|
intercellular spread of bacteria regulation of motility |
-listeria form a plasma membrane protrusion when a motile bacterium accidentally contacts the host cytoplasmic membrane. the bacterium is either returned to the same cell or taken up by a neighboring cell which cytophagizes the membrane protrusion resulting in listeria being within a double membrane phagosome
-when inside the new vacuole, listeria removes actA therby delaying motility for a short period of time beffore actA expression resumes. this delay is presumably beneficial because it increases the time the bacterium is in a new cell and hence increases the time time for replication and increases the number of bacterial offspring in each cell |

