![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
37 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
What is Matter? |
Anything that has mass and takes up space |
|
|
|
What is a Solid? |
- Fixed Shape- Fixed Volume- Particles are closely packed and vibrate |
Cube |
|
|
What is a Liquid? |
- Shape changes to fill container - Fixed Volume - Particles are close but flow |
Glass of water |
|
|
What is a Gas? |
- No definite shape - No definite volume - Particles are far apart and move quickly |
Boiling Water |
|
|
What is Expanding? |
The tendency of matter to change in volume in response to a change in temperature. When a substance is heated, gases contract and tires likely lose pressure. |
Moving from one state to another |
|
|
Define physical properties |
Can be observed without changing the identity of the substance/observe but not changing it. |
Seeing the sunset |
|
|
Define physical changes |
Changes the form of a substance without changing its identity. All the properties stay the same. |
|
|
|
Give examples of physical change |
- Cutting paper - Breaking glass - Grinding spices - Melting Ice |
|
|
|
What are chemical changes? |
One or more substance changing I to a new substance. New substance has different compositions and properties from its original form. |
|
|
|
Give examples of chemical change? |
- Rusting iron - Burning wood - Baking a cake |
|
|
|
What are signs of a chemical change? |
- Change if color - Change of odor - Formation of gas - Formation of a precipitate. |
|
|
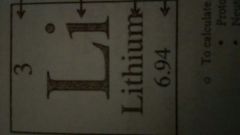
Describe the properties of the element |

3 - Atomic Number Li - Symbol Lithium - Name 6.94 - Mass |
|
|
|
Is a Neutron? a) Positive b) Neutral c) Negative |
b) Neutral |
|
|
|
Is a Proton? a) Positive b) Neutral c) Negative |
a) Positive |
|
|
|
Is a Electron? a) Positive b) Neutral c) Negative |
c) Negative |
|
|
|
Protons are... Neutrons are... Electrons are... |
Protons - Same as atomic number Neutrons - Round the atomic mass and subtract the atomic number Electrons - Same as atomic number |
|
|
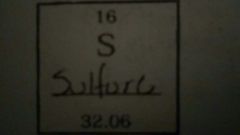
Describe the properties of the given element |
Atomic # = 16 Atomic Mass = 32.06 # of Protons = 16 # of Neutrons = 16 # of Electrons = 16 |
|
|
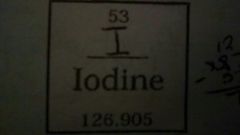
Describe the properties of the given element |
Atomic # = 53 Atomic Mass = 126.905 # of Protons = 53 # of Neutrons = 74 # of Electrons = 53 |
|
|
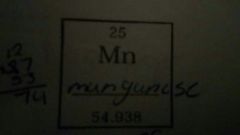
Describe the properties of the given element |
Atomic # = 25 Atomic Mass = 54.938 # of protons = 25 # of Neutrons = 30 # of Electrons = 25 |
|
|
|
Mendeleev organized the periodic table by increasing what? |
Mass and atomic number |
|
|
|
Horizontle rows on the periodic table are called what? |
Groups |
|
|
|
Vertices columns are called what? |
Periods |
|
|

Remember this... |
Ok |
|
|

Remember this... |
Ok |
|
|
|
What are valence? |
The number of electrons in the other shells. |
|
|
|
What is a element? |
Pure substance with unique sets of properties. Example: Nitrogen |
|
|
|
What is a compound? |
Chemically combined substances. Example: H2O |
|
|
|
What is a mixture? |
Substance that are not chemically combined; may or may not be able to see visibly diverse parts. Example: Salad |
|
|
|
H2 + O2 -> H2O2 What are the parts? |
2 Hydrogen + 2 Oxygen will give us Hydrogen Peroxide |
|
|
|
What is endothermic energy? |
*Absorbs heat Example: baking soda and vinegar, hot hands heating pockets. |
|
|
|
What is Exothermic energy? |
*Gives off heat Example: elephant toothpaste, making ice cubes |
|
|
|
What is a reaction where two or more substances combine to form a new compound? |
Chemical reaction |
|
|
|
What is a reaction in which a single compound breaks down to form two or more simpler substances? |
Decomposition |
|
|
|
What is a reaction in which one element takes the place of another element in a compound? |
Single replacement |
|
|
|
What is a reaction in which a compound forms from the exchange of ions between two compounds? |
Double replacement |
|
|
|
What does the Law of Conservation of Mass state? |
Mass is neither created or destroyed, it is simply rearranged. *If there are 5 atoms of Oxygen in the reactants, there are 5 atoms of Oxygen in the products. |
|
|
|
What is a substance that speeds up a reaction without it being changed? |
A Catalyst |
|

