![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
43 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
What Are The States Of Matter? |
Solid,Liquid,Gas and Plasma |
4 states |
|
|
What is Lustre? |
The way light interacts with the surface of a crystal, rock, or mineral. |
|
|
|
What is Malleability? |
It can be hammered or bent into different shapes. |
|
|
|
What Is Ductility? |
It can be pulled into wires. |
|
|
|
What Is Solubility? |
The ability of a substance to dissolve in a solvent. |
|
|
|
What is Compound? |
A substance formed when two chemical elements are chemically bonded together. |
|
|
|
What is an element? |
An element is on the periodic table of elements. |
|
|
|
What is a Homogneous mixture? |
Homogneous is where you can't see all of the different components that make it up. Eg.milk |
|
|
|
What is a mixture? |
A mixture contains at least two types of substances or two different types of particles. |
|
|
|
What is an atom? |
An atom is the smallest unit of matter that has the properties of an element. |
|
|
|
What is a molecule? |
Molecules form when two or more atoms form chemical bonds with each other. |
|
|
|
What is a Heterogeneous mixture? |
It is where you can see all of the parts that make it up. Eg.Pizza |
|
|
|
What is Evaporation? |
Evaporation is when a liquid changes state into a gas. |
|
|
|
What is condensation? |
It is where water that collects as droplets on a cold surface when humid air is in contact with it. |
|
|
|
What is Sublimation? |
A substance changes from a solid to a gas without ever passing through a liquid phase. Eg.Dry ice |
|
|
|
What is Conductivity? |
It measures the water's ability to conduct electricity. |
|
|
|
What is Precipitate? |
A precipitate is an insoluble solid that emerges from a liquid solution. The emergence of the insoluble solid from solution is called precipitation. |
|
|
|
What is the Particle Theory Of Matter? |
1. All matter is made up of tiny particles. 2. All particles of one substance are the same. Different substances are made up of different particles. 3. The particles are always moving. The more energy the particles have, the faster they move. |
|
|
|
What are some characteristics of a Metal? |
Solid,lustre,good conductor,malleable and ductile. |
|
|
|
What are some characteristics of a non metal? |
Solid, liquid or gas,dull,poor conductor,brittle and not ductile. |
|
|
|
5 indicators of Chemical Change? |
New colour,bubbles,release heat,odor and impossible to reverse. |
|
|
|
What is its called from a solid to a liquid? |
Melting. |
|
|
|
What is it called from a liquid to a gas? |
Evaporation. |
|
|
|
What is it called from a gas to a liquid? |
Condensation |
|
|
|
What is it called from a liquid to a solid? |
Freezing |
|
|
|
What are the Chemical properties of matter? |
Are properties that can be measured or observed only when matter undergoes a change to become an entirely different kind of matter. They include reactivity, flammability, and the ability to rust. Nearly impossible to change the state back to the original once changed. |
|
|
|
What's the outer circle called? |
Valence Shell |
|
|
|
What's the way to test for Oxygen? |
1.Light a wooden splint. 2.Blow out the flame but leave the splint glowing. 3.Hold the glowing splint in a small amount of the unknown gas. 4.If the splint bursts into flame,the gas is Oxygen. |
|
|
|
What's the way to test for Hydrogen? |
1.Light a wooden splint. 2.Hold the burning splint in a small amount of the unknown gas. 3.If you hear a pop sound,gas is Hydrogen. |
|
|
|
How do you test for Carbon dioxide? |
1.Bubble the unknown gas through the limewater solution. 2.Add a few drops of limewater to the gas and swirl it around. 3.If the limeeater turns cloudy or looks milky,the gas is carbon dioxide. |
|
|
|
How do you test for water vapor? |
1.Hold a could surface near the suspected water vapour. 2.Touch a piece of blue colbalt chloride paper to any liquid that condenses. 3.If the paper turns colour brom blue to pink,water is present. |
|
|
|
How do you get the atomic number? |
# of protons and electron in the atom |
|
|
|
How to get the number of Neutrons? |
Mass-Protons |
|
|
|
What's the Bohr Rutherford diagram? |

|
|
|
|
What is atomic mass and atomic number? |
Atomic # is the number it is on the periodic table. Atomic mass is the mass of the element. |
|
|
|
What's a Lewis dot diagram? |
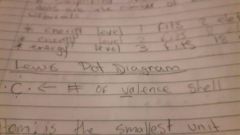
|
|
|
|
What's the rows and columns called? |
Group/family and period |
|
|
|
What is HHPS? |
Hazardous Household Product Symbols. |
|
|
|
What is WHMIS? |
Workplace,hazardous materials information system. |
|
|
|
What is an isotope? |
Forms of the same element that contain equal numbers of protons but different numbers of neutrons in their nuclei. |
|
|
|
What is an ion and how to create it? |
Ions can be created, by either chemical or physical means, via ionization. If they give away an atom then it has a positive charge. If it takes one then they have a negative charge. |
|
|
|
What is standard atomic notation? |

|
|
|
|
Name and label an atom. |

|
|

