![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
17 Cards in this Set
- Front
- Back
|
What is the dependent variable? |
The independent variable is what you measure in an experiment |
|
|
What is the control variable? |
The control variable is what you keep the same in an experiment? |
|
|
What is the independent variable? |
The independent variable is what you change in the experiment |
|
|
How is crude oil made? |
Millions of years ago the remains of dead animal and plants fall to the bottom of the ocean where over time it gets buried under by mud and soon it gets buried so deep that the intense temperature and pressure turns them into hydrocarbons-crude oil |
|
|
What is the process of catalytic cracking? |
Long chains of hydrocarbons are vaporised into gas and it is put over hot powdered aluminium oxide (catalyst) then the hydrocarbons split and falls into the surface of the catalyst |
|
|
Why do we use catalyst cracking? |
It is to split useless long chains of hydrocarbons into shorter and more useful hydrocarbons which are in high demand |
|
|
What are the properties of short chain hydrocarbons? |
Shorter chains are more flammable, less viscous,more volatile (how easy it is to turn into a gas), lower boiling points and have a low carbon to oxygen ratio during combustion |
|
|
What are the properties of long chain hydrocarbons? |
They are less flammable, more viscous, less volatile, higher boiling points and have a high carbon to oxygen ratio during combustion |
|
|
Name the first 4 Alkanes |
Methane Ethane Propane Butane |
|
|
Name the first 4 Alkenes |
Methene Ethene Propene Butene |
|
|
What is the formulae for Alkanes? |
CnH2n+2 |
|
|
What is the formulae for Alkenes? |
CnH2n |
|
|
What is the formulae for Alcohols? |
CnH2n+1OH |
|
|
What is the formulae for Carboxylic Acids? |
CnH2n+1COOH |
|
|
Name the first 4 types of alcohols? |
Methanol Ethanol Propanol Butanol |
|
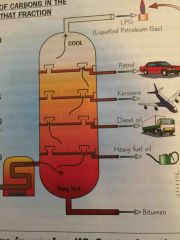
What is fractional distillation? |
It is a way of separating hydrocarbons from crude oil and putting them in different fractions |
|
|
What is the process of fractional distillation? |
The crude oil is vaporised and put through a fractionating column which has a temperature gradient where it’s hot at the bottom and cold at the top. The short chain HC would condense nearer the top and the longer chain HC would condense nearer the bottom |

