![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
45 Cards in this Set
- Front
- Back
|
Homogeneous Heterogeneous |
Ho- uniform He- not uniform |
|
|
Letters are related to words just like ____ are related to compound |
Elements |
|
|
Why compounds are classified as a ps |
All 1 type of matter w/ consistent properties |
|
|
Length Volume Temp |
M L K |
|
|
-1Gm -1 uL -1mm -1 ng -1 Mb |
- 10*9 -10*-6 -10*-3 -10*-9 -10*6 |
|
|
Acid rain corroding Satie of Liberty |
Chemical change |
|
|
Dissolving salt in water |
Physical change |
|
|
Melting butter |
Physical change |
|
|
Boiling water |
Physical change |
|
|
Burning gasoline |
Chemical change |
|
|
Burning gasoline |
Chemical change |
|
|
Freezing Mercury |
Physical change |
|
|
Gold |
Element and pure substance |
|
|
Gold |
Element and pure substance |
|
|
Dishwater |
Mixture and Ho or He |
|
|
Gold |
Element and pure substance |
|
|
Dishwater |
Mixture and Ho or He |
|
|
Calcium Metal |
Element and pure substance |
|
|
Water |
Compound and pure substance |
|
|
Water |
Compound and pure substance |
|
|
Milk |
Mixture and Ho |
|
|
Water |
Compound and pure substance |
|
|
Milk |
Mixture and Ho |
|
|
Vitamin C |
Compound and Pure substance |
|
|
Water |
Compound and pure substance |
|
|
Milk |
Mixture and Ho |
|
|
Vitamin C |
Compound and Pure substance |
|
|
Why does ice melt slower in salt water then in fresh water? |
Ice melts and cold fresh water floats on dense salt water making ice melt slower. |
|
|
Viscosity |
How well a liquid flows |
|
|
Viscosity |
How well a liquid flows |
|
|
Malleability |
Ability of solid to be shaped |
|
|
Viscosity |
How well a liquid flows |
|
|
Malleability |
Ability of solid to be shaped |
|
|
Density |
Ratio of mass to volume |
|
|
Similarities and differences btw the melting point and freezing point |
Similarity: same temp Difference: solid~liquid(melting) Liquid~solid(freezing) |
|
|
When substance melts. What happens to Motion of the molecules of the the substance? |
Speed up |
|
|
How boiling point can help identify what type of substance you may have |
Different boiling points for each substance |
|
|
How boiling point can help identify what type of substance you may have |
Different boiling points for each substance |
|
|
How chemical change is different from chemical change |
Change in identity of matter ( chemical bond) |
|
|
How boiling point can help identify what type of substance you may have |
Different boiling points for each substance |
|
|
How chemical change is different from chemical change |
Change in identity of matter ( chemical bond) |
|
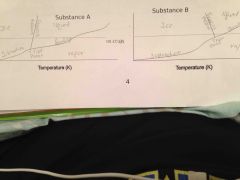
Which substance will frees eat a HIGHER temp when the pressure is increased? |
Substance A |
|
|
During chemical reactions what is being broken and formed? |
Chemical bonds |
|
|
Chemical reaction equation |
Reactants➡️products |
|
|
3 possible clues that indicate that a chemical reaction might have occurred |
1. Gas produced 2. Solid produced 3. Color change |

