![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
40 Cards in this Set
- Front
- Back
|
What is iron used for?
|
Ideal catalyst for oxidation-reduction reactions
Heme – much of iron is in red blood cells (1 mg Fe/1 ml packed RBCs) |
|
|
How is iron stored/Distributed in body
|
1. Hemoglobin 67%
2. Storred as ferritin (water soluble), hemosiderin (water insoluble), 27% 3. myoglobin 5% 4. Iron requiring proteins 1% (cytochroms, iron sulfur clusters, enzymes using non-heme iron) comes in and little is lost |
|
|
Normal iron in RBC?
in body? Life of RBC? |
1. rbc- 2.7 g
2. 3-5 g in body 3. 120 destroyed then macrophages grab iron released |
|
|
Two types of iron?
|
Ferrous 2+
Ferric 3+ (dietary) |
|
|
How does iron transport? (name transporters)
|
– Ferrireductase
– DMT1 (divalent transporter-1) – HEPHAESTIN – FERROPORTIN |
|
|
Dietary iron (Fe+3) is reduced to Fe2+ by what?
|
DCytb (duodenal cytochrom-like b)
|
|
|
Describe the transport process of iron in enterocyte...
|
1. Fe +3 is reduced by Dcytb to Fe+2
2. DMT-1 transports Fe+2 on apical surface of enterocyte in microvili region |
|
|
What happens to mouse without DMT1?
|
microcytic anemia (no iron in RBCs)
|
|
|
Describe the transport of iron out of enterocyte picking up after it was brought in my DMT1...
|
1. iron moves to basolateral side of enterocyte
2. transported out by ferroportin, which requires a ferroxidase (Hephaestin) to help 3. Once out amount absorbed in body and liver controlled by hepcidin |
|
|
1. What carries iron to the tissues and in what form?
2. How does iron get into erythroblast? |
1. Fe +3 carried by transferrin
2. (TfR) transferrin receptor mediated endocytosis |
|
|
Describe the importance of the kiss and run hypothesis
|
Endosome docks onto mitochondria and iron is removed because mitochondria is more acidic causing TfR to hold ferritin tightly but lets go of iron
|
|
|
Protein involved in reducing Ferric to ferrous Fe after it leaves endosome...
|
Steap3 (ferrirductase)
|
|
|
What transports iron out of an endosome?
|
DMT1
|
|
|
What are main causes for iron deficiency?
|
1. Insufficient Dietary iron
2. Menstruating women 3. Aspirin abuse 4. Ulcers of the GI tract (Blood loss) 5. Hypochromic microcytic anemia |
|
|
Describe the two main causes of iron overloading...
|
1. Genetic causes: when things are wrong with the transport system and Fe absorption is increased (hereditary hemochromatosis)
2. Conditional: repeated blood transfusions (sickle cell anemia_ |
|
|
What is the clinical definition of HHI
|
Organ dysfunction due to iron overload: cirrhosis, arthritis, endocrinopathy, skin pigmentation, cardiomyopathy; usually manifests in 6th decade
|
|
|
Genetic definition of HH1?
a. inheritance b. gene c. functional probs |
a. AR 1/250 MC AR in man
b. HFE gene, Cys 282--> Tyr (C282Y) secondary mutation is His 63 Asp (H63D) c. Malregulation of iron uptake and export by enterocyte |
|
|
What tests are used to diagnose HH?
|
serum ferritin (protein storage of iron) >400ng/ml (normal is 20-300)
- CT, MRI, or histo exam of liver ( |
|
|
What does hepcidin do as far as regulatory roles?
|
Negative controller
binds to ferroportin and destroys it - without ferroportin can not bring iron into the rest of the body |
|
|
What causes Hepcidin levels to increase or decrease (assuming no pathology)?
|
Hfe via signal transduction
1. Hfe binds normally to Tfr1 and when there are higher levels of iron Hfe bound to Tfr1 are replaced by iron and then bind to hepcidin signaling complex (TfR2) 2. Via signal transduction (Smad pathway) induces hepcidin expression and prevents iron expression |
|
|
Explain how a mutation in Hfe leads to iron overloading
|
mutated Hfe cannot bind to TfR2 and therefore hepcidin cannot be expressed and ferroportin cannot be regulated leading to iron overload
|
|
|
Besides Hfe there are a few other ways HH can be caused.... what are they?
|
1. A77D (auto dominant): Alanine to aspartic acid inactivates ferroportin, which increases iron in enterocytes and macrophages
2. Val 162 deletion: ferroportin hepcidin interactions is disabled 3. TfR2 mutation (Auto recessive) leaves inability to release hepcidin |
|
|
What gene(s) is/are found mutated in juvenile hemochromatosis?
|
HJV- (hemojuvelin) which is part fo signaling pathway to hepcidin
could also have Rare (HAMP mutation) hepcidin antimicrobial peptide |
|
|
Gene/chromosomes mutated in the types of hemochromatosis?
a. Type I (classical) b. Type II (Juvenile) c. Type III d. Type IV |
a. Hfe chr 6
b. HJV- chr. 1 and sometimes HAMP- Chr. 19 c. TfR2- Chr 7 d. Ferroportin def. chr. 2 |
|
|
Which protein is being looked at as possible treatment for iron and why?
|
Tmprss6 due to fact that it downregulates hepcidin transcription, and therefore could be used in iron overload therapies
|
|
|
What is role of Ttc7 and the idea in role of iron problem treatments?
|
causes loss of iron and may be used in future HH1 treatment
|
|
|
What vitamins does the production of RBCs depend on?
|
Vit B12 (cobalamin) and folate (folic acid
|
|
|
Deficiencies in B12 and folate can cause sever anemia called?
|
megaloblastic anemia
|
|
|
What causes megaloblastic anemia?
|
slowed synthesis of DNA in in developing RBC in bone marrow and a nuclear maturation defect (which is mandatory for RBC to be released)
Decreased B12 or folate prevents syntesis of adequate amounts of DNA |
|
|
What lab findings with megaloblastic anemia?
|
Large erythrocytes
MCV > 100fL (norma= 80-100) MCHC (mean cell hemoglobin content)= normal |
|
|
What is clinical presentation of megaloblastic macrocytic anemia
|
lethargy weakness, yellow or waxy pallor, loss of weight and appetite, diarrhea
Also neuro- pins and needles (peripheral neuropathy) |
|
|
|
|
|
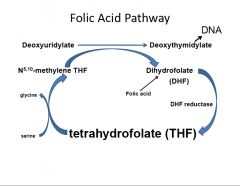
Describe in words folate metabolism to DNA synthesis... Also how does it all start up again...?
|
1. Key players... (N5) of THF and serine (transfers carbon kicks N5,10 methylene-THF)
2. Carbon of N5,10 methylene-THF transferred to deoxyuridylate (dUMP) and makes deoxythymidylate (dTMP), which is used to make DNA 3. Dihydrofolate (DHF) is formed as a biproduct of #2 and is reduced by dehydrofolate reductase to THF |
|
|
What are the two proteins required for transport of folate in cells?
|
1. High affinity folate receptor- concentrates folate in vescicles
2. membrane folate transporter- transfers from vescicle to cytosol |
|
|
What does N5-methyl- THF need to enter folic acid cycle as THF?
|
Vit. B12 (cobalamin) - demythylates it
|
|
|
B12 aka cobalamin is transported by what in the blood?
|
transcobalamin
|
|
|
|
|
|
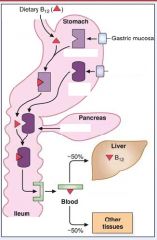
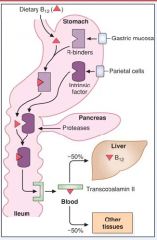
Describe in steps the absorption of dietary B12...
|
1. B12 binds to R-binders made by gastric mucosa
2. IF (made from parietal cells of stomach) carry B12 to ileum after B12 is released from R-binder protein which was degraded by proteases from pancreas 3. Receptors (cubulin) in the ileum bring B12 to body |
|
|
Lack of IF can cause what?
|
pernicious anemia due to failure to absorb vitamin B12
- parietal cells in stomach fail to make it |
|
|
What is gold standard to determine if it is IF or just B12 that is deficient?
|
Schilling test
|

