![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
208 Cards in this Set
- Front
- Back
|
What is a normal PO2 value?
|
In young adults the range is between 85-100 mmHg.
This value decreases with age; at 60 years the average is about 85 mmHg. |
|
|
What shifts an oxygen dissociation curve to the right?
|
Increase in temperature
Increase in PCO2 Increase is H+ Increase in 2,3-DPG |
|
|
What are four primary causes of reduced PO2 in arterial blood?
|
1. Hypoventilation
2. Diffusion impairment 3. Shunt 4. Ventilation-perfusion inequality |
|
|
Hypoventiliation:
|
- Means that the volume of fresh gas going to the alveoli per unit time is reduced. If the resting oxygen consumption is not correspondingly reduced, hypoxemia results.
- Hypoventilation ALWAYS causes a rise in PCO2, and this is a valuable diagnostic feature. - Hypoxemia can easily be abolished by increasing the inspired PO2 by delivering oxygen via a face mask. |
|
|
Sleep apnea:
|
1) central: there are no respiratory efforts
2) obstructive: despite activity of the respiratory muscles there is no airflow. |
|
|
Central sleep apnea:
|
Often occurs in patients with hypoventilation because respiratory drive is depressed during sleep.
During REM sleep, breathing is often irregular and unresponsive to chemical and vagal drives. An exception is hypoxemia, which usually remains a powerful stimulus to breathe. |
|
|
Obstructive sleep apnea:
|
Common in obese patients, airway obstruction can be caused by backward movement of the tongue, collapse of the pharyngeal walls, greatly enlarged tonsils or adenoids, and other anatomical causes of pharyngeal narrowing.
Loud snoring often occurs, and the patient may wake violently after an apneic episode. Chronic sleep deprivation may lead to daytime somnolence, impaired cognitive function, chronic fatigue, morning headaches and personality disturbances. Treatment is by continuous positive airway pressure (CPAP) by means of a facemask during sleep. |
|
|
Some causes of hypoventilation:
|
1. Depression of the respiratory center by drugs (barbituates and morphine)
2. Disease of the medulla 3. Abnormalities of the spinal cord or anterior horn 4. Disease of the nerves to the respiratory muscles (guillain barre or diptheria) 5. Diseases of the myoneural junction (myasthenia gravis, anticholinesterase poisoning) 6. Diseases of the repsiratory muscles (progressive muscular dystrophy) 7. Thoracic cage abnormalities (crushed chest) 8. Upper airway obstruction (tracheal compression by a thymoma) |
|
|
Diffusion impairment:
|
This means that equilibriation does not occur between the PO2 in the pulmonary capillary blood and alveolar gas.
In normal persons, at rest and during exercise, there is plenty of time for blood flowing through the lungs to equilibriate with alveolar gas. In some diseases, the blood-gas barrier is thickened and diffusion is so slowed that equilibriation may be incomplete. Hypoxemia could also result from an extreme reduction in contact time. For example, a large pulmonary embolus can divert so much blood flow from regions of a lung that the time for oxygenation within the capillary is reduced to 1/10 normal. Hypoxemia caused by diffusion impairment can be corrected by administering 100% oxygen. CO2 elimination is usually not impaired; typically the arterial PCO2 is slightly lower than normal because ventilation is overstimulated, either by the hypoxemia or by intrapulmonary stretch receptors. |
|
|
Shunt:
|
A shunt allows some blood to reach the arterial system without passing through ventilated regions of the lung.
In addition, an unventilated but perfused area of lung constitutes a shunt. Many shunts are extrapulmonary, including those that occur in congential heart disease through atrial or ventricular septal defects or a patent ductus arteriosus. If a patient with a shunt is given 100% O2, the arterial PO2 fails to rise to a normal level. (This is ONLY seen in shunts!) Shunts do not cause PCO2 to increase because it is countered by chemoreceptors which increase ventilation if the PCO2 increases. Often the arterial PCO2 is lower than normal because of the additional hypoxemic stimulus to ventilation. |
|
|
Ventilation-Perfusion Inequality:
|
In this condition, ventilation and blood flow are mismatched in various regions of the lung, with the result that all gas transfer becomes inefficient.
This cause of hypoxemia is extremely common; it is responsible for most of the hypoxemia of COPD, interstitial lung disease and vascular disorders like pulmonary embolism. A reduction is CO can cause a fall of PO2 in mixed venous blood, which results in a fall of arterial PO2 for the same degree of ventilation-perfusion inequality. (This situation may be seen in patients who develop an MI with mild pulmonary edema) To determine if a patient has a ventilation-perfusion inequality we must figure out the alveolar-arterial difference for PO2. |
|
|
Normal values for PCO2:
|
The normal arterial PCO2 is 37-43 mm Hg and is uneffected by age.
It tends to fall slightly during exercise and rise slightly during sleep. |
|
|
What are two causes of CO2 retention?
|
1) hypoventilation
2) ventilation-perfusion inequality |
|
|
Hypoventilation as a cause of increased PCO2:
|
Whereas the hypoxemia of hypoventilation can be relieved easily by increasing the inspired PO2, the CO2 retention can only be treated by increasing the ventilation. This may require mechanical assistance.
|
|
|
Ventilation-perfusion inequality as a cause of increased PCO2:
|
Chemoreceptor response to increased PCO2 raise the ventilation rate.
The result is that the arterial PCO2 is returned to its normal level, but the arterial PO2 does not return all the way to normal. Some patients that have increased work of breathing may not compensate by increasing respiratory rate. |
|
|
What is a normal arterial pH?
|
7.4
|
|
|
Respiratory acidosis:
|
This is caused by CO2 retention.
It is important to distinguish between acute and chronic CO2 retention. Chronic forms compensate for a fall in pH by retaining more bicarbonate at the kidney. |
|
|
Metabolic acidosis:
|
This is caused by a fall in bicarbonate; due to diabetic ketoacidosis or lactic acidosis.
A fall in arterial pH stimulates the peripheral chemoreceptors, increasing ventilation and lowering PCO2. |
|
|
Respiratory alkalosis:
|
This is seen in acute hyperventilation.
If hyperventilation is maintained, the kidney may compensate by excreting bicarbonate. |
|
|
Metabolic alkalosis:
|
This is seen in disorders such as severe prolonged vomiting when the plasma bicarbonate concentration rises.
Often there is no respiratory compensation but sometimes PCO2 rises slightly. Metabolic alkalosis also occurs when a patient with long-standing lung disease and compensated respiratory acidosis is ventilated too vigorously, thus rapidly bringing the PCO2 down. |
|
|
Measurement of diffusing capacity:
|
Measured by determing the input versus output of a single breath containing a known value of CO.
|
|
|
Causes of reduced diffusing capacity for carbon monoxide:
|
Blood-gas barrier
- Thickened in interstitial lung disease - Area is reduced in emphysema and pulmonectomy Capillary blood - Volume reduced in pulmonary embolism - Concentration of red cells reduced in anemia |
|
|
Quick review of acid-base disturbances:
|
Respiratory acidosis:
PCO2 goes up and HCO3 goes up to compensate Metabolic acidosis: HCO3 goes down and PCO2 goes down to compensate Respiratory alkalosis: PCO2 goes down and HCO3 goes down to compensate Metabolic alkalosis: HCO3 goes up and usually the lungs do nothing to compensate. |
|
|
Heart disease caused by thiamine deficiency:
|
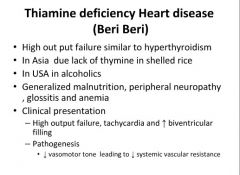
|
|
|
Clinical signs of Beri Beri:
|
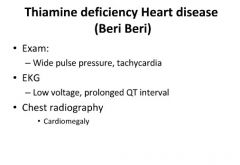
|
|
|
Heart disease with hemochromatosis:
|

|
|
|
Histology of hemochromatosis in heart disease:
|
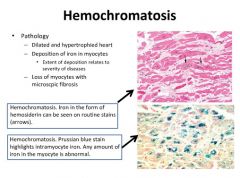
|
|
|
Symptoms of hemochromatosis:
|
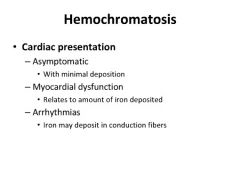
|
|
|
Diagnosis and treatment of hemochromatosis:
|

|
|
|
Sarcoidosis is a systemic disease:
|

|
|
|
Sarcoidosis in heart disease:
|

|
|
|
Histology of cardiac sarcoidosis:
|
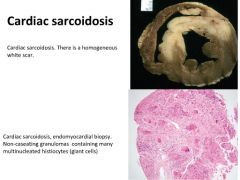
|
|
|
Pulmonary sarcoidosis induced cor pulmonale:
|
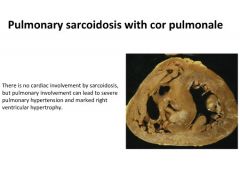
|
|
|
Diagnosis of cardiac sarcoidosis:
|
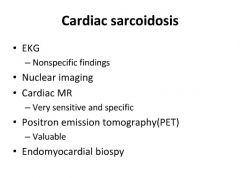
|
|
|
Drug induced cardiac disease:
|

|
|
|
Diseases in which the Functional Residual Capacity (FRC) and Residual Volume (RV) are increased:
|
Diseases in which there is an increased airway resistance, for example, emphysema, chronic bronchitis and asthma.
|
|
|
Diseases in which the Functional Residual Capacity (FRC) and Residual Volume (RV) are reduced:
|
Often seen in patients with reduced lung compliance, for example, in diffuse interstitial fibrosis. In this case, the lung is stiff and tends to recoil to a smaller resting volume.
|
|
|
How lung elasticity is measured:
|
By generating a pressure vs volume curve using a balloon passed through the nose or esophagus.
- Curves for emphysema and asthma are shifted upward and to the left. - Curves for rheumatic valve disease and interstitial fibrosis are flattened. * Elastic recoil is reduced in patients with emphysema; the pressure volume curve is moved up and to the left as a result of the destruction of the alveolar walls (leading to air trapping). * Elastic recoil is increased in interstitial fibrosis, thus reducing the lung's distensibility. |
|
|
How airway resistance is measured:
|
Airway resistance is measured as the pressure difference between the alveoli and the mouth divided by the flow rate.
* Airway resistance in reduced by an increase in lung volume because the expanding parenchyma exerts traction on the airway walls. * Airway resistance is increased in chronic bronchitis (due to excessive secretions) and emphysema (airways lose traction of tissues surrounding them due to destruction of the alveolar walls) * Airway resistance is also increased in patients with bronchial asthma; due to contraction of bronchial smooth muscle, mucous plugs and edema. * Tracheal obstruction increases airway resistance. This may be caused by compression from an enlarged thyroid or by narrowing or scarring or a tumor. An important feature is that the obstruction is usually apparent during inspiration and an audible stridor may be present. |
|
|
Measurement of control of ventilation:
|
- The ventilatory response to CO2 can be measured with a rebreathing technique. A small bag is filled with a mixture of 6-7% CO2 with oxygen, and the patient rebreathes from this over a period of minutes. The bag PCo2 increases at the rate of 4-6 mm Hg/min because of the CO2 being produced from the tissues, and thus the change in ventilation per mm Hg increase in PCO2 can be determined.
The ventilatory response to hypoxia can be measured in a similar way. A bag is filled with 24% O2, 7% CO2 and the balance N2. During rebreathing, the PCO2 is monitored and held constant. As oxygen is taken up, the ventilation is related to the PO2 in the bag and the lungs. Both of these techniques give information about the overall ventilatory response to CO2 or hypoxia, but do not differentiate between patients who will not breathe because of CNS or neuromuscular inadequacy and those who cannot breathe because of mechanical abnormalities of the chest. To make this distinction, the mechanical work done during inspiration can be measured. |
|
|
Interpretation of control of ventilation:
|
The ventilatory response to CO2 is depressed by sleep, narcotic drugs and genetic, racial and personality factors.
The factors that affect the ventilatory response to hypoxia are less understood. The response is reduced in persons who have been hypoxemic since birth, such as those born at high altitude or with cyanotic congenital heart disease. The hypoxic ventilatory response tends to be preserved in sleep. However, some patients develop sleep apnea syndromes. |
|
|
Measurement of lung function with exercise tests:
|
The normal lung has enormous reserves of function at rest. For example, the O2 uptake and CO2 output can be increased 10-fold during exercise, and these increases occur without a fall in arterial PO2 or a rise in PCO2.
To reveal minor dysfunction, the stress of exercise is often useful. Another reason for exercise testing is to assess disability. Patients vary in their own assessment of the amount of activity they can do, and an objective measurement can be revealing. Occasionally, exercise tests are diagnostic, for example, in exercise induced asthma and in myocardial ischemia causing angina. Exercise tests can help evaluate the cause of dyspnea. In most instances, the interpretation of the tests during exercise is similar to that of tests done at rest except that exercise exaggerates the abnormalities. |
|
|
Dyspnea:
|
Refers to the sensation of difficulty with breathing. Dyspnea occurs when the demand for ventilation is out of proportion to the patient's ability to respond to that demand. As a result, breathing becomes difficult, uncomfortable or labored.
An increased demand for ventilation is often caused by changes in the blood gases and pH level. A reduced ability to respond to the ventilatory needs is generally caused by abnormal mechanics of the lung or chest wall. Frequently, increased airway resistance is the problem, as in asthma, but other causes include a stiff chest wall. |
|
|
Topographic measurements of lung function:
|
The regional distribution of blood flow and ventilation in the lung can be measured with radioactive substances. A major application of this method in practice is the diagnosis of pulmonary embolism.
|
|
|
Distribution of blood flow and ventilation in the lungs:
|
- Blood flow in an upright lung is uneven, being much greater at the base than at the apex. Exercise results in a more uniform distribution because of the increase in pulmonary arterial pressure; the same result is found in disease conditions such as pulmonary hypertension and left-to-right cardiac shunts.
- The distribution of ventilation is also gravity dependant, and normally ventilation to the base exceeds that to the apex. The alveoli at the base are smaller and inflate more easily, while the alveoli at the apex are larger and are more difficult to inflate. |
|
|
Catagories of pulmonary diseases:
|

The two cardinal symptoms of respiratory disease are cough and shortness of breath.
|
|
|
Mnemonic for the etiologies of pulmonary disease:
|

|
|
|
What is tidal volume?
|
The volume inspired or expired with each normal breath
|
|
|
What is inspiratory reserve volume?
|
Is the volume that can be inspired over and above the tidal volume. It is used during exercise.
|
|
|
What is expiratory reserve volume?
|
Is the volume that can be expired after the expiration of a tidal volume.
|
|
|
What is the residual volume?
|
Is the volume that remains in the lungs after a maximal expiration.
**Cannot be measured with spirometry** |
|
|
What is dead space?
|
Anatomic dead space:
- Is the volume of the conducting airways - Is normally about 150 mL Physiological dead space: - Is defined as the volume of the lungs that does not participate in gas exchange. - Is the same as anatomic dead space in normal lungs - May be greater than the anatomic dead space in lung diseases in which there are ventilation/perfusion (V/Q) defects. |
|
|
Diagram of lung volumes and capacities:
|
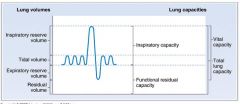
- Inspiratory capacity: the sum of tidal volume and IRV.
- Functional residual capacity: Is the sum of ERV and residual volume. Is the volume remaining in the lungs after a tidal volume is expired. Includes the residual volume, so cannot be measured by spirometry. - Vital capacity: is the sum of tidal volume, IRV and ERV. Is the volume of air that can be forcibly expired after a maximal inspiration. - Total lung capacity: Is the sum of all four lung volumes. Is the volume in the lungs after a maximal inspiration. Includes residual volume, so cannot be measured by spirometry. |
|
|
Diagram of FEV and FVC in asthma and fibrosis:
|
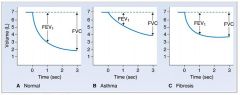
- FEV1 is the volume of air that can be expired in the first second of a forced maximal expiration.
- FEV1 is normally 80% of the forced vital capacity |
|
|
How much lung function is lost when a patient gets short of breath doing daily activities?
|
At least half of the lung function.
|
|
|
X-ray of patient with obstructive lung disease:
|
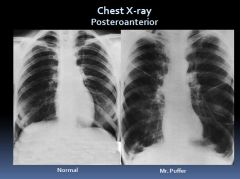
In obstructive lung disease, air gets in but can’t get out so you see hyperinflation.
Note that his diaphragm is “scalloped” and flattened. |
|
|
Lung mechanics in obstructive lung disease:
|
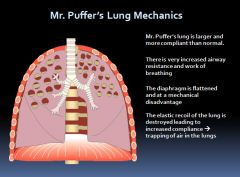
|
|
|
Diagram explaining Vmax:
|
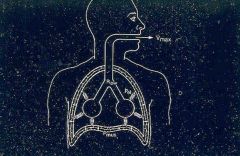
Vmax (maximum exhalation velocity) is related to 3 forces.
Sum of the muscular pressures and the elastic recoil = the driving force pushing air out of the lungs |
|
|
Diagram explaining the equal pressure point in the airways:
|
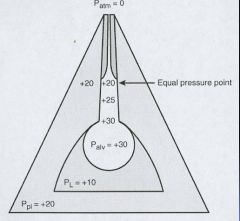
Pleural pressure tends to stay the same
As air moves up the airways, the air pressure will equalize to the pleural pressure. This tends to occur in airways lined with cartilage. If the elastic recoil is reduced, pleural pressure remains the same but the total air pressure is reduced and this changes the equal pressure point to a lower part of the airway – leading to collapse of lungs structures NOT lined by cartilage. (An increase in airway resistance or a decrease in lung compliance leads to collapse of airways) |
|
|
Airway anatomy:
|
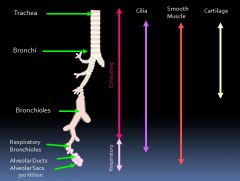
|
|
|
Normal airway equal pressure point:
|
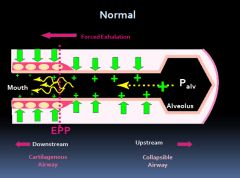
|
|
|
Equal pressure point in obstructive lung diseases:
|
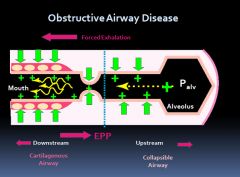
|
|
|
Why do patients with obstructive lung disease breath with pursed lips?
|
Because it helps prevent the collapse of the upstream airways.
|
|
|
Diagram of alveolar ventilation events:
|
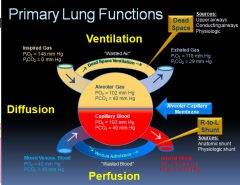
|
|
|
Mediastinal Anatomy:
|
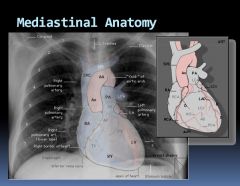
|
|
|
Mediastinal anatomy on an x-ray:
|
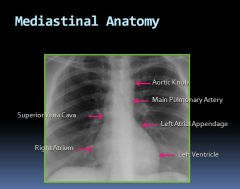
|
|
|
Hilar anatomy on an x-ray:
|
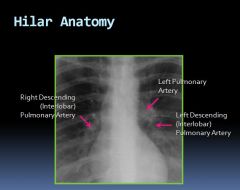
|
|
|
Central airway anatomy on an x-ray:
|
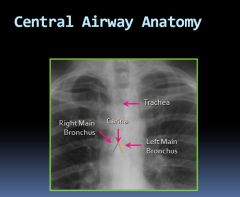
|
|
|
Normal cardiothoracic ratio:
|
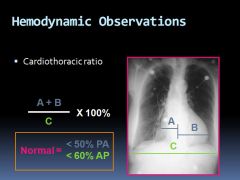
|
|
|
X-ray of a pericardial effusion:
|
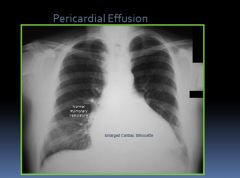
|
|
|
X-ray of an endotrachial tube:
|
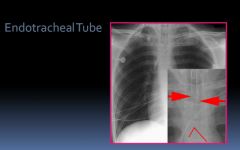
- Radiopaque stripe for identification
- Tip ideally 3-5 cm above carina - Fifth thoracic vertebra typically overlies carina |
|
|
Chest tube placement:
|
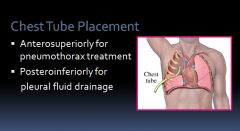
|
|
|
Lobar pneumonia on PA X-ray:
|
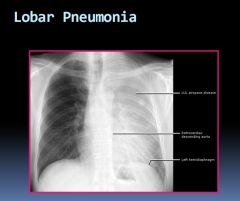
Heart border is obliterated due to pneumonia in the left upper lobe. Can still see the diaphragm because the left lower lobe
is not involved. |
|
|
What might be a cause of hoarseness?
|
A growth in the left upper lobe of the lung stretching or irritating the recurrent laryngeal nerve.
|
|
|
Normal values for arterial blood gases:
|

Tachypnea and hyperventilation are not the same.
The way to determine if someone is hyperventilating is to look at the PCO2. |
|
|
Dalton's Law:
|

|
|
|
Facts needed for Dalton's Equation:
|

|
|
|
Alveolar Air Equation:
|

NEED TO KNOW
|
|
|
How the alveolar air equation works:
|
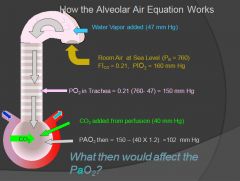
There are 5-6 causes of hypoxemia; decreased air pressure (colorado), right to left shunts, diffusion abnormalities, ventilation-perfusion abnormalities, dead space
|
|
|
V/Q relationships:
|
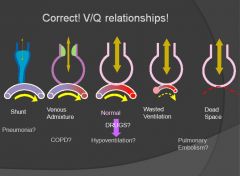
Pure hypoventilation can result in hypoxemia – BUT still have a normal A-a gradient.
|
|
|
PO2 to Oxygen saturation level relationships:
|

40 is mixed venous blood and is 75% saturated.
30 is the dropoff point, where you can get steep declines in saturation with each decrease in PO2. |
|
|
Oxygen dissociation curve:
|
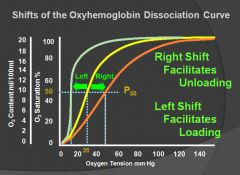
|
|
|
Relationship between PCO2 and hypoventilation:
|

|
|
|
How to tell if a pH disorder is metabolic or respiratory?
|
- If PaCO2 and serum HCO3 are moving in the same direction as the pH, it is a primary metabolic disorder.
- If PaCO2 and serum HCO3 are moving in opposite directions as the pH, it is a primary respiratory disorder. - If the PaCO2 or HCO3 remain normal in face of abnormal pH or levels abnormal with near normal pH it is a mixed disorder. |
|
|
Respiratory vs. Metabolic disorder equations:
|

*If the mechanism is being compensated for – the PCO2 should be in this calculated range.
|
|
|
Buffering of acute and chronic respiratory acidosis:
|

|
|
|
Metabolic acidosis:
|
- Net gain in acid or net loss of bicarbonate
- Can be anion gap or non-anion gap - Can have both at the same time |
|
|
Anion Gap:
|
- Serum Na+, Cl-, and HCO3-
- Na - (Cl + HCO3) difference between unmeasured cations and anions in the blood - Normal AG 12 ± 2 - Unmeasured cations: calcium and magnesium - Unmeasured anions: negatively charged proteins(albumin), phosphates, sulfates, citrate (ketones) |
|
|
Mnemonic for causes of anion gap metabolic acidosis:
|

|
|
|
Causes of non-anion gap metabolic acidosis:
|

*Carbonic anhydrase inhibitors reduce bicarbonate reabsorption in the proximal tubule.
|
|
|
Metabolic alkalosis:
|
- Net gain of alkali or loss of acid
- Low urine chloride: Saline Responsive Vomiting NG tube suction Diuretic use Post hypercapnea Achlorhydria |
|
|
Causes of acute respiratory acidosis:
|
CNS depression from drugs
CVA Neuromuscular disease-myasthenia gravis Acute airway obstruction Severe pneumonia Lung injury-flail chest Ventilator malfunction |
|
|
Causes of chronic respiratory acidosis:
|
- COPD with CO2 retention
- Chronic respiratory center depression - Pickwickian syndrome - Chronic neuromuscular disorders such as ALS and spinal cord injury |
|
|
Causes of acute respiratory alkalosis:
|
- Anxiety
- Drugs-salicylates, progesterone, catecholamines - Acute PE, hypoxia - Acute pneumonia - Sepsis - Mechanical ventilation - CNS lesion-acute CVA |
|
|
Causes of chronic respiratory alkalosis:
|
- Pregnancy: normal PaCO2 is 30-32, if you see a normal PaCO2 in late pregnancy, the patient potentially has impending respiratory failure,
- Cirrhosis, hepatic encephalopathy |
|
|
Winter's Formula:
|
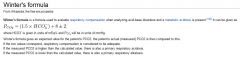
Use serum bicarbonate: the bicarbonate from the ABG is a calculated number, not measured
|
|
|
Factoid about L/min of O2:
|
** For every liter per minute of oxygen you give someone, you increase the O2 by 2%. So with 8 L/min you would add 16% to the already 21% of room air.
|
|
|
Normal Flow Volume Loop:
|
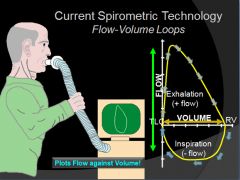
As more air is expired the airways tend to clamp down and flow out is reduced.
|
|
|
Diagnosis of an obstructive lung disease using a flow-volume loop:
|
- The primary criterion for diagnosing obstructive lung disease by spirometry is a reduced FEV1/FVC ratio.
- FEV1 is reduced disproportionately more than FVC, resulting in an FEV1/FVC ratio <70%-80% The severity of obstruction is based on the percentage of reduction in FEV1 **Basically, a normal person should be able to get out 70-80% of their lung capacity within the first second of blowing** |
|
|
Checking for reversibility of lung disease with a flow-volume loop:
|
- Spirometry is performed before and after the inhalation of a bronchodilator (albuterol)
- A significant response is defined as a >12% increase in FEV1 or FVC (or an absolute improvement in FEV1 of ≥200 mL) |
|
|
Graph of Lung Volume vs. Air Flow in restrictive and obstructive disease:
|
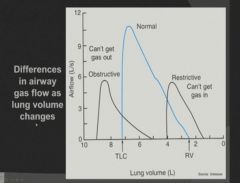
|
|
|
Flow Volume Loop seen in obstructive lung disease:
|
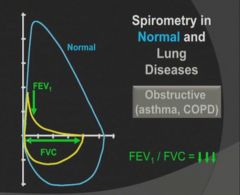
|
|
|
Flow Volume Loop seen in restrictive lung disease:
|
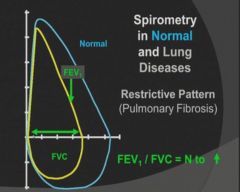
|
|
|
4 methods of determining lung volumes:
|
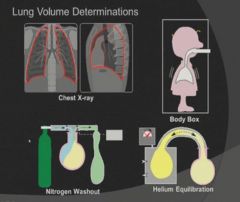
|
|
|
Helium Dilution method of measuring functional residual capacity:
|

Using C1V1 = C2V2 equation, solving for V2 to represent patients lung capacity
|
|
|
Method of measuring diffusing capacity:
|
- Assesses the ability of the lungs to take up oxygen (O2) and remove carbon dioxide (gas exchange)
- Once inhaled, O2 binds to hemoglobin in the bloodstream and is then distributed to the vital organs - Carbon monoxide (CO) is chosen for DLCO testing for convenience because it binds to hemoglobin approximately 210 times more readily than O2 |
|
|
Changes in diffusing capacity:
|

|
|
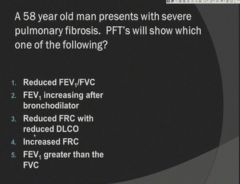
|
Reduced FRC and reduced DLCO.
|
|

|
Residual Volume
|
|
|
Endotrachial tube complications:
|

|
|
|
Central venous catheters and complications:
|
- Catheter tip typically positioned in superior vena cava above the right atrium.
- Venous malposition (Aberrant catheter tip in internal jugular, azygous, superior intercostal, contralateral subclavian, and axillary veins) - Intra-arterial malposition (midsternal or left paravertebral location) |
|
|
Tension pneumothorax:
|
- Tension pneumothorax occurs when air is able to enter but not exit the pleural space.
- Ipsilateral hemithorax hyperinflation. - Contralateral mediastinal shift - Downward displacement of diaphragm (deep sulcus sign in supine patient) |
|
|
X-ray of a tension pneumothorax:
|
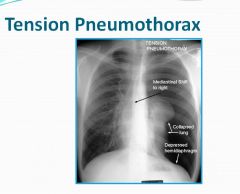
|
|
|
Atelectasis:
|
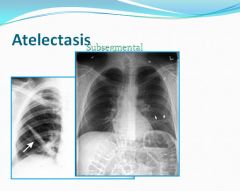
Failure of a small segment of lung to inflate.
|
|
|
Right upper lobe atelectasis:
|
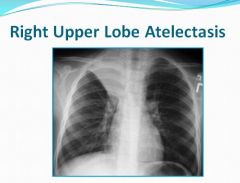
|
|
|
Right middle lobe atelectasis:
|
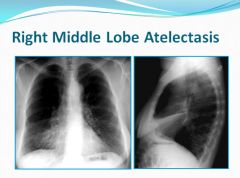
Loss of the silhouette sign of the right heart border seen on PA film
Fissure seen on lateral film |
|
|
Right lower lobe atelectasis:
|
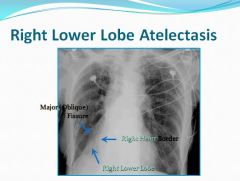
|
|
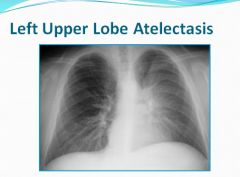
Left upper heart border is obliterated and there is a haze.
|
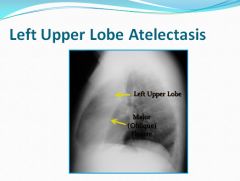
lateral view
|
|
|
Left lower lobe atelectasis:
|
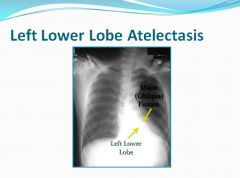
Distinct line behind the heart can be seen.
|
|
|
Viral Pnuemonia:
|
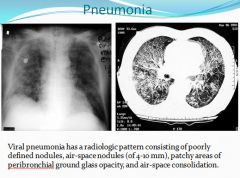
|
|
|
Emphysema on x-ray:
|
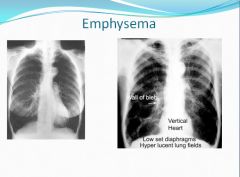
"Blebs" are cysts.
|
|
|
Three conditions that cause increased resistance to airflow:
|
1) Blockage of the lumen by secretions or foreign material
2) Contraction of the bronchial smooth muscle, hypertrophy of the mucous glands and inflammation and edema of the wall 3) Destruction of lung parenchyma may cause loss of radial traction leading to narrowing. A bronchus may also be compressed by an enlarged node or neoplasm. Peribrochial edema can also cause narrowing. |
|
|
COPD:
|
Chronic obstructive pulmonary disease encompasses patients who have emphysema, chronic bronchitis or a mixture of the two.
|
|
|
Emphysema:
|
- Emphysema is characterized by enlargement of the air spaces distal to the terminal bronchiole, with destruction of their walls.
- In centriacinar emphysema, the destruction is confined to the terminal and respiratory bronchioles. (tends to start in the apex) - In panacinar emphysema, the peripheral alveoli are also involved. (no regional preference) - May be caused by excessive amounts of the enzyme lysosomal elastase released from neutrophils in the lung. This results in the destruction of elastin and type IV collagen. Smoking may stimulate macrophage released neutrophil attractants, or inhibit elastase inhibitors such as a1-antitrypsin. |
|
|
Chronic Bronchitis:
|
- This disease is characterized by excessive mucous production in the bronchial tree, sufficient to cause excessive expectoration of sputum. (most days for at least 3 months in the year for at least the last two years)
- The hallmark is hypertrophy of mucous glands in the large bronchi. Excessive amounts of mucous are found in the airways, and semisolid plugs of mucous may occlude some small bronchi. Small airways are narrowed and inflammed and bronchial smooth muscle increases. - Smoking is the cause of most cases; air pollution from smog or industrial smoke may also be a factor. |
|
|
Type A COPD (pink puffers):
|
Most common in middle aged men.
- Increasing dyspnea over the years - Little or no cough - Marked chest overexpansion - No cyanosis - Quiet breath sounds - Normal JVP - No peripheral edema - Arterial PO2 only slightly depressed - Arterial PCO2 normal |
|
|
Type B COPD (blue bloater):
|
Most common in middle aged men.
- Increasing dyspnea over the years - Increasing cough with expectoration, increasing in severity over the years - Moderate or no increase in chest volume - Worsening SOB - Often cyanosis - May have rales or rhonchi - May have increased JVP - May have peripheral edema - Will have pulmonary congestion - PO2 often very low - PCO2 often raised |
|
|
Asthma:
|
- This disease is characterized by increased responsiveness of the airways to various stimuli and is manifested by inflammation and widespread narrowing of the airways that changes in severity, either spontaneously or as a result of treatment.
- There will be hypertrophied smooth muscle, edema, mucous gland hypertrophy and extensive infiltration by eosinophils and lymphocytes. |
|
|
Atelectasis:
|
- Atelectasis is loss of lung volume caused by inadequate expansion of air spaces. It results in shunting of inadequately oxygenated blood from pulmonary arteries into veins, thus giving rise to a V/Q imbalance and hypoxia.
- Resorption atelectasis: Occurs when an obstruction prevents air from reaching distal airways. The air already present is gradually absorbed, and alveolar collapse follows. The most common cause of resorption collapse is obstruction of a bronchus by a mucous or mucopurulent plug. - Compression atelectasis: Is usually associated with accumulations of fluid, blood or air within the pleural cavity, which mechanically collapse the adjascent lung. This is a frequent occurance with pleural effusions. Leakage of air into the pleural cavity also leads to compression atelectasis. - Contraction atelectasis: Occurs when either local or generalized fibrotic changes in the lung or pleura hamper expansion and increase elastic recoil during expansion. * Contraction atelectasis is not reversible |
|
|
Acute lung injury:
|
- Encompasses a wide spectrum of pulmonary lesions.
- Clinically manifests as acute onset of dyspnea, decreased arterial oxygen pressure, development of bilateral pulmonary infiltrates on radiographs and absence of clinical evidence of primary left-side heart failure. - Acute lung injury can progress to the more severe acute respiratory distress syndrome |
|
|
Acute Respiratory Distress Syndrome overview:
|
- Caused by diffuse alveolar capillary and epithelial damage. There is usually rapid onset of life-threatening respiratory insufficiency, cyanosis and severe arterial hypoxemia that is refractory to oxygen therapy and that may progress to multisystem organ failure. The histological manifestation of ARDS in the lungs is known as diffuse alveolar damage.
- Can occur as a result of direct injury to the lungs or indirect injury in the setting of a systemic process. |
|
|
Pathogenesis of ARDS:
|
- In ARDS, the integrity of the microvascular endothelium and alveolar epithelium is compromised by either endothelial or epithelial injury, or more commonly, both.
- This damage causes increased vascular permeability and alveolar flooding, loss of diffusion capacity and surfactant abnormalities due to damaged type II pneumocytes. - Lung injury is caused by an imbalance of pro-inflammatory and anti-inflammatory factors. Damaged cells release IL-8, IL-1 and TNF to recruit neutrophils to the area. Activated neutrophils release products that damage the epithelium and maintain the inflammatory cascade. This process could be counter-acted by endogenous antiproteases, antioxidants and anti-inflammatory cytokines that are upregulated by pro-inflammatory cytokines...In the end it is the balance between destruction and protection that determines the degree of tissue injury and severity of ARDS. |
|
|
Clinical course of ARDS:
|
- Most patients develop ARDS within 72 hours of the initiating insult.
- Prognosis is grim, with mortality rates between 60-100% - Predictors of poor prognosis are advanced age, underlying sepsis and development of multi-organ failure. - If the patient survives the acute stage, diffuse interstitial fibrosis may occur and compromise respiratory function. But most patients get normal lung function back in 6-12 months. |
|
|
Diffuse Interstitial (Restrictive, Infiltrative) Lung Diseases:
|
- A heterogenous group of disorders characterized predominately by diffuse and usually chronic involvement of the pulmonary connective tissue, principally the most peripheral and delicate interstitium in the alveolar walls.
- The hallmark of these disorders is reduced compliance; more pressure is required to expand the lungs because they are stiff, which in turn causes increased effort of breathing (dyspnea). - V/Q mismatches lead to hypoxia. - The earliest common manifestation of most interstitial diseases is alveolitis; accumulation of inflammatory and immune effector cells within the alveolar walls and spaces. - With persistant injury, cellular interactions involving lymphocytes, macrophages and neutrophils lead to parenchymal injury, proliferation of fibroblasts, and progressive interstitial fibrosis. Activation of pulmonary macrophages is a key event in the pathogenesis of interstitial fibrosis. - Macrophages recruit and activate neutrophils which injure epithelial cells and degrade connective tissue. Macrophages attract fibroblasts and contribute to fibrosis. |
|
|
Idiopathic pulmonary fibrosis:
|
- A pulmonary disorder of unknown etiology characterized by diffuse interstitial fibrosis, which in advanced cases results in severe hypoxemia and cyanosis.
- Males are effected more often than females and mostly at an age older than 60. - The histologic pattern of fibrosis is referred to as "usual interstitial pneumonia". - The lungs have a cobblestoned appearance because of retraction of scars. White rubbery fibrosis is seen with lower lobe predominance and distinctive distribution in the subpleural regions and along the interlobular septa. - IPF presents insidiously with the gradual onset of nonproductive cough and progressive dyspnea. Most patients will have dry or velcro like lung sounds. Cyanosis, cor pulmonale and peripheral edema develop in later stages. - Diagnosis requires lung biopsy. - Mean survival without lung transplant is 3 years or less. |
|
|
Nonspecific Interstitial Pneumonia:
|
- Patients with NSIP have a diffuse interstitial lung disease of unknown etiology wherin the lung biopsies fail to show diagnostic features of any of the other interstital diseases.
- Has a worse prognosis than usual interstitial pneumonia. - Patients with a cellular pattern have a better outcome than those with the fibrosing pattern. |
|
|
Cryptogenic Organizing Pneumonia:
|
- Patients present with cough and dyspnea and radiographically have subpleural or peribronchial patchy areas of airspace consolidation.
- Histologically, this is characterized by the presence of polypoid plugs of loose organizing connective tissue within alveolar ducts, alveoli and often bronchioles. - Some individuals recover spontaneously, but most require treatment with oral steroids for at least 6 months. |
|
|
Summary of diffuse interstitial fibrosis:
|
- Diffuse interstitial fibrosis of the lung gives rise to restrictive lung diseases characterized by reduced lung compliance and reduced forced vital capacity. Ratio of FEV to FVC is normal.
- The diseases that cause diffuse interstitial fibrosis are heterogenous. The unifying pathogenic factor is injury to the alveoli with activation of macrophages and release of fibrogenic cytokines such as TFG-B - Idiopathic pulmonary fibrosis is prototypic of restrictive lung diseases. It is characterized by patchy lung fibrosis and formation of cystic spaces (honeycomb lung). This histologic pattern is known as "usual interstitial pneumonia" |
|
|
Pneumoconioses:
|
- Describes the non-neoplastic lung reaction to inhalation of mineral dusts (coal, silica and asbestos).
- These almost always result from workplace exposure; but asbestos exposure may effect family members as well. - The reaction of the lungs depends on the size of the particle; particles that are 1 to 5 micrometers are the most dangerous, because they get lodged at the bifurcation of the distal airways. Macrophages that reside at bifurcations endocytose the particles leading to release of products that promote inflammation, fibroblast proliferation and collagen deposition. - Some inhaled particles may reach the lymph system and initiate an immune response to either the particles themselves or particle modified proteins. This can lead to amplification and extension of the local reaction. * Tobacco smoking worsens the effects of all inhaled mineral dusts, more so with asbestos than with any other particle. |
|
|
Coal Worker's Pneumoconiosis:
|
- Pulmonary anthracosis is the most innocent coal induced pulmonary lesion and is also commonly seen in all urban dwellers and tobacco smokers. Carbon pigment accumulates in the connective tissue along the lymphatics and in lymph nodes.
- Simple CWP is characterized by coal macules and the somewhat larger coal nodule. The coal macule and nodule consist of dust laden macrophages and collgen fibers. Lesions are scattered throughout the lung; upper lobes are more heavily involved. - Complicated CWP: occurs on a background of simple CWP by coalescence of coal nodules and generally requires many years to develop. It is characterized by intensely blackened multiple scars larger than 2 cm. - Clinical course: CWP is usually benign. In those who develop complicated CWP, there is increasing pulmonary dysfunction, pulmonary hypertension and cor pulmonale. Unfortunately, complicated CWP has a tendency to progress even in the absence of further exposure. |
|
|
Silicosis:
|
- THe most common chronic occupational disease in the world.
- After inhalation the particles interact with macrophages and epithelial cells. Ingested silica particles cause activation and release of mediators by macrophages; IL-1, TNF, free radicals, etc... - TNF is the major mediator - Inhalation of pure quartz silica is worse than inhalation of a mixed silica - Start as tiny nodules in the upper lobe with hyalinized collagen fibers creating a "whorled" appearance. - As the disease progresses, the nodules may coalesce into hard scars, with eventual progression to PMF (pulmonary massive fibrosis) - Radiographs show a fine nodularity in the upper lobes but pulmonary function is usually normal. - SOB develops late in the disease along with PMF - development of pulmonary hypertension and cor pulmonale - Silicosis is associated with an increased susceptibility to tuberculosis. |
|
|
Asbestosis:
|
- Occupational exposure to asbestos is linked to:
1) parenchymal interstitial fibrosis 2) localized fibrous plaques or diffuse fibrosis in the pleura 3) plueral effusions 4) bronchogenic carcinoma 5) malignant pleural and peritoneal mesotheliomas 6) laryngeal carcinoma - Amphibole (straight, stiff and brittle fibers) type of asbestos is the worst. - Asbestosis, like other pneumoconioses, causes fibrosis by interacting with lung macrophages. - Asbestos also acts as a tumor initiator and promotor; smoking causes absorption of carcinogens in smoke onto the fibers and is synergistic in the development of bronchogenic carcinoma. |
|
|
Histology and clinical course of asbestosis:
|
- Same fibrotic changes seen in other diseases.
- Will see asbestos bodies; golden brown, fusiform or beaded rods with a translucent center. - Begins in the LOWER lobes and moves upward a disease progresses. - Contraction of fibrous tissue may created honeycombing and fibrosis of pleura may stick the lungs to the chest wall. - May see serous or bloody pleural effusions. **Pleural plaques are the most common manifestation of asbestos exposure; dense collagen often containing calcium found on the parietal pleura over the diaphragm. - Clinical findings are the same as other fibrotic diseases. - Worsening SOB will appear 10-20 years after exposure with a productive cough - Increased risk of normally rare mesotheliomas - Greatly increased risk of bronchogenic carcinoma with concommitent smoking |
|
|
Drug and radiation induced pulmonary disease:
|
- Bleomycin, an anticancer agent, causes pneumonitis and interstitial fibrosis as a result of direct toxicity of the drug and by stimulating the influx of inflammatory cells into the alveoli.
- Amiodarone, an anti-arrythmic agent, also causes pneumonitis and fibrosis - Acute radiation pneumonitis occurs 1-6 months after therapy in 20% of patients and is manifested by fever, dyspnea, pleural effusion and pulmonary infiltrates in the area of radiation. These symptoms may resolve with corticosteroids or progress to chronic radiation pneumonitis, associated with pulmonary fibrosis. |
|
|
Sarcoidosis epidemiology and pathogenesis:
|
- Sarcoidosis is a multisystem disease of unknown etiology characterized by noncaseating granulomas in many tissues and organs.
- Bilateral hilar lymphadenopathy or lung involvement on radiograph is the major presenting manifestation; eye and skin involvement may occasionally be the presenting symptom - More common in adults under 40, Danish, Swedish and US AA's and non-smokers. - Probably driven by a CD4+ Th1 cell mediated response to an unknown antigen; local secretion of IFN-g and IL-2 induce T cell proliferation and activation of macrophages. - May be genetically influenced; some association with HLA-A1 and HLA-B8 |
|
|
Sarcoidosis histology and clinical course:
|
- The histologic finding in sarcoidosis is the noncaseating epithelioid granuloma; a collection of epithelioid cells and giant cells rimmed by CD4+ T cells.
- Other manifestations include lymph node enlargment, eye involvement, skin lesions (erythema nodosa, lupus pernio), and liver, skin and marrow involvement. Lung involvement occurs is most cases. - Patient may be asymptomatic. - If not....gradual appearance of respiratory symptoms and constitutional symptoms. - Disease has unpredictable course; may progress or remiss - only 10-15% succumb to progressive pulmonary fibrosis and cor pulmonale. |
|
|
Hypersensitivity pneumonitis:
|
- An immunologically mediated inflammatory lung disease that primarily affects the alveoli.
- Most often caused by an occupational allergen such as mold. - Presents as a predominately restrictive lung disease with decreased diffusion capicity, lung compliance and total lung volume. - Bronchioalveolar lavage specimens show increased numbers of CD4 and CD8 T cells - May present as either acute or chronic |
|
|
Pulmonary eosinophilia:
|
A number of entities are characterized by an infiltration and activation of eosinophils by IL-5.
- Acute eosinophilic pneumonia with respiratory failure: rapid onset of fever, dyspnea, hypoxia and pulmonary infiltrates - responds to cortiocosteroids. - Simple pulmonary eosinophilia - Tropical eosinophilia: caused by infection with microfilariae - Secondary eosinophilia: in association with asthma, drug allergies and certain forms of vasculitis - Idiopathic chronic eosinophilic pneumonia: aggregates of lymphocytes and eosinophils within septal walls and alveolar spaces, accompanied by high fever, night sweats and dypsnea. |
|
|
Smoking related interstitial diseases:
|
- Desquamative interstitial pneumonia (DIP): accumulation of large numbers of macrophages with dusty brown pigment in the airspaces; alveolar septa are thickened by inflammatory infiltrate and there is mild interstitial infiltrate. Pulmonary functions show a mild restrictive abnormality. Good prognosis with steroid therapy and smoking cessation.
- Respiratory bronchiolitis: Characterized by pigmented macrophages as in DIP, but in a bronchiolocentric pattern. Mild peribronchiolar fibrosis is also seen. As with DIP, patients present with gradual onset of dyspnea and dry cough, and the symptoms recede with smoking cessation. |
|
|
Calcuation of A-a gradient:
|
A-a gradient = PAO2 − PaO2[2]
Where: PAO2 = alveolar PO2 (calculated from the alveolar gas equation) PaO2 = arterial PO2 (measured in arterial blood A-a gradient) In general the A-a gradient can be calculated by: Aa Gradient = [FiO2*(Patm-PH2O)-(PaCO2/0.8) ] - PaO2 On Room air ( 21 % ) and at sea level, a simplified version of the equation is: Aa Gradient = (150 - 5/4(PCO2)) - PaO2 |
|
|
Asthma:
|
- Asthma is an inflammatory disease with thickening of the bronchial wall
- Type I hypersensitivity reaction - IgE mediated |
|
|
Immediate response to allergen in asthma:
|
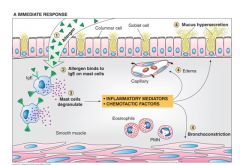
- Allergan reacts with IgE attached to mast cells located in themucosal surface and in the mucosa.
- Mast cell degranulation and release of inflammatory mediators: Leukotreines, Ach, Histamine, Prostaglandin D2, PAF - Bronchoconstriction, vasodilation/edema, mucus hypersecretion - Chemotactic factors attract Eosinophils, basophils, mast cells |
|
|
Delayed response (4-8 hours) in asthma:
|
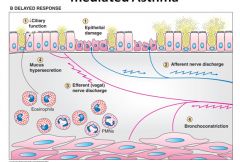
- Eosinophils mediate decreased ciliary function and epithelial cell necrosis.
- Recruitment of additional inflammatory cells - Stimulation of autonomic nerves - End result: the mucosa is inflamed and edematous, mucus hypersecretion, bronchospasm |
|
|
Histology of asthma:
|
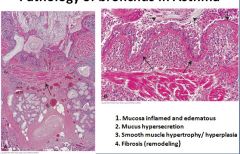
Fibrosis in the sub-epithelial layer
Lungs are enlarged due to trapping of air Abnormalities will be seen in bronchioles where constriction of the airways is controlled Smooth muscle constriction happens in all levels that contain muscle, but is most important at the level of the bronchiole |
|
|
Histology of asthma part 2:
|
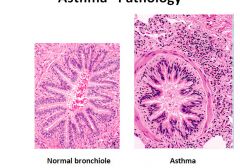
Eosinophils are heavily involved in asthma. May see some eosinophils in the lumen or even some crystals from degranulation.
Smooth muscle band in the asthma picture is hypertrophied and prominent. |
|
|
Fatal asthma:
|
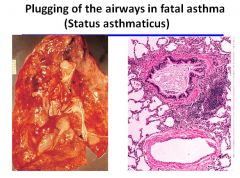
|
|
|
Overview of COPD:
|
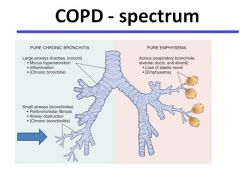
|
|
|
Pathology of chronic bronchitis:
|

|
|
|
Overview of changes to airways in chronic bronchitis:
|
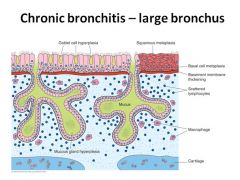
|
|
|
Histology of chronic bronchitis:
|
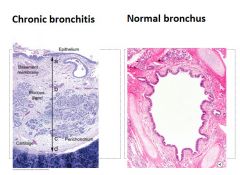
Glandular hypertrophy and hyperplasia with fibrosis and inflammatory cells
|
|
|
Pathology of chronic bronchitis in the small airways:
|

|
|
|
Pathogenesis of small airway obstruction:
|
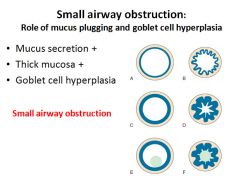
|
|
|
Emphysema:
|
- Destruction and enlargement of air spaces distal to terminal bronchiole:
Respiratory bronchiole Alveolar ducts Alveoli |
|
|
Histology of emphysema:
|
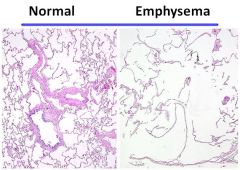
|
|
|
Two types of emphysema:
|
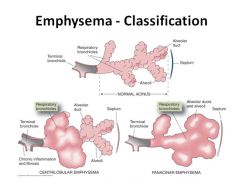
|
|
|
Centriacinar vs. panacinar emphysema:
|
Centriacinar means only some of the “unit” involved.
Panacinar means that the entire “unit” is involved. |
|
|
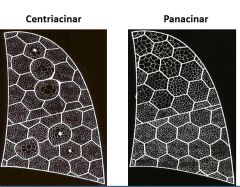
Distribution of centriacinar vs. panacinar emphysema:
|
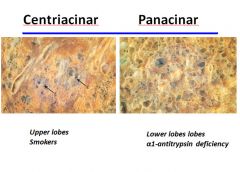
|
|
|
Pathogenesis of emphysema:
|
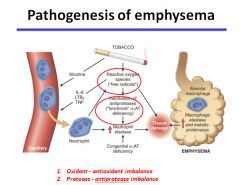
|
|
|
Alveolar collapse in emphysema:
|
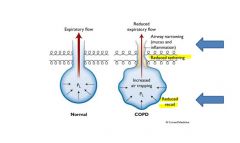
. The lower flows seen in chronic obstructive pulmonary disease (COPD) patients result from both decreased pressure due to the loss of elastic recoil and increased resistance due to airway narrowing. Pressure decreases as elastic recoil is lost. The decrease in elastic recoil is thought to be caused by increased elastin degradation and by slightly reduced elastin content although an altered arrangement of the elastic tissue in the COPD patients’ lung, rather than total elastin, may account for the observed decrease. Increased airway resistance in COPD patients is caused by narrowed airways. In COPD, the average cross-sectional area of the smallest bronchioles is about one-sixth of that seen in normal lungs. Narrowed airways in the range of 0.2 to 0.4 mm are associated with air trapping and result in increased residual volume/total lung capacity ratios. Inflammation leading to fibrosis and intraluminal mucus is thought to be a primary cause of airway narrowing.
|
|
|
Right heart failure in COPD:
|
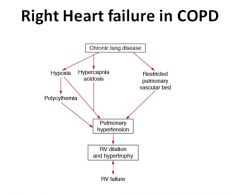
The evolution of right ventricular (RV) failure in chronic obstructive airways disease (chronic bronchitis and emphysema; chronic obstructive pulmonary disease). The factors on the left arise primarily from the bronchitis; those on the right from emphysema
|
|
|
COPD vs. Asthma:
|
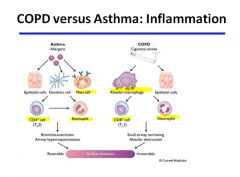
|
|
|
COPD vs. Asthma histology
|
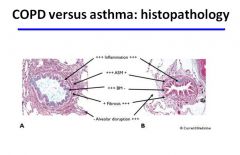
Differences in the histopathology of chronic obstructive pulmonary disease (COPD) and asthma. A, Appearance of a small airway from a patient who died of an asthma attack. B, Small airway from a patient with severe COPD. In both airways there are increased numbers of inflammatory cells. The basement membrane (BM) is thickened in asthma (subepithelial fibrosis) but not in COPD. Airway smooth muscle (ASM) is markedly thickened in asthma as a result of hypertrophy and hyperplasia, but in COPD there is little increase as compared to normal conditions. A small degree of fibrosis may be seen in the airway wall in asthma, but in COPD there is increased fibrosis of the airway wall, especially around the adventitia (peribronchiolar fibrosis).
|
|
|
Systemic inflammation in COPD:
|
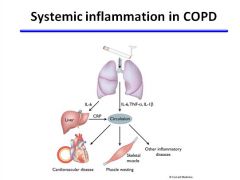
Systemic features of chronic obstructive pulmonary disease (COPD) and comorbidities. Inflammation in the lungs may spill over into the systemic circulation, resulting in systemic inflammatory effects and exacerbation of cardiovascular diseases and skeletal muscle wasting. Increased concentrations of interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) are found in the systemic circulation of patients who have severe COPD; these concentrations may directly result in skeletal muscle atrophy. IL-6 releases the acute phase protein C-reactive protein (CRP), which is associated with increased cardiovascular disease risk. Cigarette smoke also contributes to increased cardiovascular risk.
|
|
|
Epidemiology of COPD:
|
- COPD is now the 3rd leading cause of death in the United States (behind heart disease and cancer).
- Only disease in top 3 that is increasing in prevalence. - Higher prevalence in men but more women die now of COPD than men in US-death rates for women up 30% from 1980-2000 |
|
|
Clinical findings for emphysema:
|
DESTRUCTION OF LUNG TISSUE
SHORTNESS OF BREATH ON EXERTION PROGRESSES WITH AGE/SMOKING MAY BE NOTED ON CHEST X RAY NO COUGH IRREVERSIBLE |
|
|
Diagnostic tests for COPD:
|
- PULMONARY FUNCTION TESTING
MOST HELPFUL PAINLESS NON-INVASIVE GIVES IMPORTANT PROGNOSTIC INFORMATION - CHEST X RAY LIMITED VALUE |
|
|
Loss of lung function over time:
|
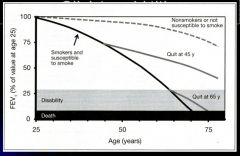
- EVERYONE LOSES LUNG CAPACITY YEARLY AFTER MID-20’S FROM AGING
- SUSCEPTIBLE SMOKERS LOSE 3X LUNG CAPACITY YEARLY - WHEN YOU STOP YOU IMMEDIATELY GO TO THE NON-SMOKERS RATE OF LOSS - SPIROMETRY –HELPFUL SCREENING TOOL |
|
|
COPD graph of volume vs. time:
|
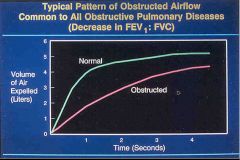
End of the line is the FVC and at the 1 second mark is the FEV1
|
|
|
Clinical manifestations of COPD:
|
INCREASED DYSPNEA
COUGH/SPUTUM ANXIETY - OVERUSE OF MEDS/OXYGEN IMMOBILIZATION NUTRITIONAL DECLINE ALTERED MENTAL STATUS |
|
|
Assessment of COPD severity 1:
|

|
|
|
Assessing severity of COPD 2:
|

|
|
|
Causes of hypoxemia:
|
LOW FI02
ALTITUDE-BAROMETRIC PRESSURE V/Q INEQUALITY HYPOVENTILATION DIFFUSION IMPAIRMENT |
|
|
Indicators that a patient needs to be hospitalized:
|

|
|
|
Overview of asthma:
|

Diagnosis of asthma:
- Episodic symptoms of airflow obstruction or airway hyperresponsiveness are present - Airflow obstruction is at least partially reversible, measured by spirometry - Alternative diagnoses are excluded |
|
|
History to get when diagnosing asthma:
|

Some asthmatics are sensitive to NSAID’s.
Cortisol production drops overnight so attacks in the middle of the night are indicative of asthma. |
|
|
Common triggers of asthma:
|

|
|
|
Physical Exam findings in asthma:
|

|
|
|
Alternative diagnoses to consider other than asthma:
|

|
|
|
The alveolar air A-a gradient equation, again:
|
At sea level and breathing room O2, the alveolar O2 is:
PAO2 = (Pbarometric – PH2O) * .21 – (PCO2/.8) |
|
|
Definition of ARDS:
|
A syndrome of acute respiratory failure characterized by noncardiogenic pulmonary edema manifested by severe hypoxemia caused by right-to-left shunting through collapsed or fluid-filled alveoli
|
|
|
Clinical features of ARDS:
|

|
|
|
Chest X-ray of ARDS patient:
|
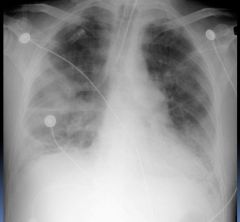
Diffuse
Patchy Interstitial Inhomogeneous No CHF |
|
|
ALI vs. ARDS:
|
In ALI: A PaO2/FiO2 ratio of less than or equal to 300 mmHg, regardless of support
In ARDS: Hypoxia is more severe, with a PaO2/FiO2 ratio less than or equal to 200 mmHg, regardless of support |
|
|
1st Phase of ARDS:
|

|
|
|
Histology of the exudative phase of ARDS:
|
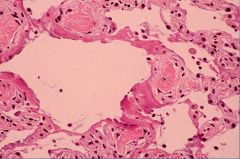
Exudative 1st phase: alveoli become filled with exudative fluid
Loss of volume Inflammatory cells Thickened A/C membrane High protein fluid Hyaline membrane Type I cell injury |
|
|
CT scan of ARDS patient:
|
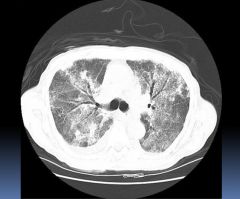
Ground glass appearance; consolidated lung with patent bronchi
|
|
|
2nd and 3rd phase of ARDS:
|

|
|
|
Histology of 3rd phase fibrosis in ARDS:
|
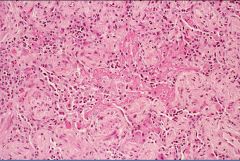
3rd phase: fibrosis is irreversible and may lead to permanent long term restrictive lung disease – but most patients will not develop this and will be normal a year later.
|
|
|
Definition of Lung Failure:
|

|
|
|
Causes of ALI/ARDS:
|
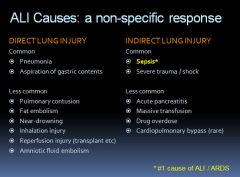
|
|
|
Diagram of pathology involved in ARDS:
|
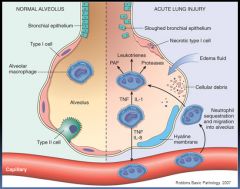
|
|
|
Treatment of ARDS:
|

*The pressure in the lung when it is inflated.
- The level of PEEP (low, medium or high) does not appear to affect the patient outcome – have to use best judgment. “ Keep the fluids down, and the tidal volume low” is the strategy of choice to treat ARDS. - If hypercapnia and acidosis -increase VR as high as 35 b/min |
|
|
Survivors of ARDS may experience:
|

|
|
|
Uses of a hyperbaric oxygen chamber:
|
Can raise the PO2 to 2000!
- Used for carbon monoxide poisoning. - May be used for severe anemic crisis - Gas gangrene infections - Decompression sickness |
|
|
Why do some COPD patients develop CO2 retention on oxygen therapy?
|
- The ventilatory drive in COPD patients is stimulated by hypoxia; removing hypoxia will decrease the ventilation rate.
|
|
|
Damage to the lungs related to oxygen therapy:
|
- Exposure to 100% oxygen may cause increased permeability in the capillary endothelial cells leading to pulmonary edema.
- Substernal pain occurs after breathing 100% O2 for 24 hours - After 36 hours a progressive fall in arterial PO2 is seen. - Concentrations of 50% or higher for more than 2 days may produce toxic changes. - It is important to avoid oxygen toxicity because the only way to relieve the resultant hypoxemia is by raising the inspired oxygen, thus creating a vicious cycle. |
|
|
Atelectasis and oxygen therapy:
|
- If an airway becomes occluded while a patient is being given oxygen, atelectasis will develop much more rapidly than normal
- This is because the nitrogen usually present in the alveoli slows reabsorption of gas is gone due to due increased oxygen delivery --> predisposition of the lung to collapse. * Absorption atelectasis is common in patients with respiratory failure because they often have excessive secretions of cellular debris in their airways and they are frequently treated with high oxygen concentrations. - Lung units with low ventilation-perfusion ratios may become unstable and collapse when high oxygen mixtures are inhaled --> increased shunting |
|
|
What is Retrolental Fibroplasia?
|
- Fibrosis behind the lens of the eye in newborn infants with respiratory distress syndrome treated with high concentrations of oxygen.
- Avoided by keeping the arterial PO2 below 140 mmHg |

