![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
82 Cards in this Set
- Front
- Back
|
When do you suspect a metabolic disorder?
|
When the clinical presentation does not fit the traditional medical textbook definition, does not respond to common treatment, or defies the clinical rational.
|
|
|
A patient can have a chronic unrecognized metabolic disorder that only becomes obvious during?
|
Physical stress, fasting or acute illness/ injury. Not recognizing a metabolic disorder or delaying treatment can result in irreversible injury to the brain, major organs, or death.
|
|
|
Lysosomal Storage Disease
|
Acid hydrolases breakdown macromolecules
LSD results from lack of any protein essential for the normal function of lysosomes. |
|
|
Gangliosidoses
|
Type of neuronal storage disease
Caused by the accumulation of gangliosides (abundant in the brain) within neurons |
|
|
GM2 Gangliosidoses
|
Caused by deficiency of lysosomal enzymes:
|
|
|
Deficiency in Hexoaminidase A causes
|
Tay-Sachs Disease
|
|
|
Deficiency in Hexosaminidase B causes
|
Sandhoff Disease
|
|
|
Deficiency in activator protein causes
|
BM2 gangliosidosis, variant AB
|
|
|
Storage Disorder vs. Poisoning
|
Accumulation of active metabolites is poisoning.
Accumulation of inert substrates/metabolites is a Storage Disorder. |
|
|
Lysosome
|
Releases digestive enzymes into mitochondria to break down its macromolecules
|
|
|
Tay-Sachs Disease
|
- high incidence in Ashkenazi Jews
- Gene on chromosome 15, > 100 mutation |
|
|
How do you diagnose Tay Sachs
|
Enzyme assay of serum, WBC, or cultured fibroblasts
|
|
|
Clinical S/S of Tay-Sachs Disease
|
- Normal at birth
- 6 mo - psychomotor retardation - Blindness - Motor incoordination, eventual flaccidity, mental deterioration - Eventual decerebrate state - Cherry RED SPOT in MACULA - Death by 2-3 years |
|
|
Pathology of Tay-Sachs Disease (Brain)
|
Brain: Normal/little/big depending upon duration.
Survival > 2 years; brain is big |
|
|
Pathology of Tay-Sachs Disease (Microscopy)
|
- Enlarged ballooned neurons filled with PAS positive material (stored gangliosides)
- Storage also in other brain cells (astrocytes and microglia) - EM - membranous cytoplasmic bodies |
|

|
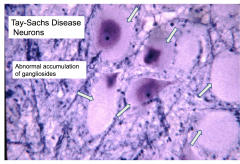
Abnormal accumulation of gangliosides
|
|
|
Treatment of Tay-Sachs
|
Still in experimental stages
"Chaperone" proteins may help the alpha subunit to fold normally. Enzyme replacement therapy |
|

|
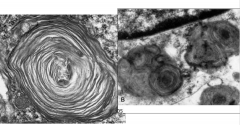
Tay-Sachs Disease with membranous cytoplasmic bodies
|
|
|
Krabbe's Disease
|
Lysosomal Storage Disease
Autosomal Recessive - Gene Chr 14 - Deficiency of galactocerebroside-B-galactosidase - Enzyme deficiency causes accumulation of toxic compound that injures oligodendrocytes - Galactocerebroside is a component of myelin sheaths; it accumulates in the "Globoid Cells" - Both CNS and PNS affected Dx: enzyme assay of WBC or cultured fibroblasts. |
|
|
Clinical Course of Krabbe's Disease
|
- Normal development
- Onset between 3-6 months - Irritability, development ceases - Deterioration of motor function - Tonic Spasms - Eventual opisthotonic posture - Myotonic jerking - Optic atrophy, blindness - CSF protein elevated |
|
|
Treatment of Krabbe's Disease
|
Umbilical cord/bone marrow transplantation (in pre-symptomatic phase)
Death by approx 2 years |
|

|
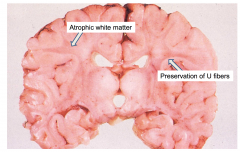
Krabbe's Globoid Cell Leukodystrophy
Atrophic brain, firm white matter, atrophic white matter with preservation of "U" fibers |
|

|
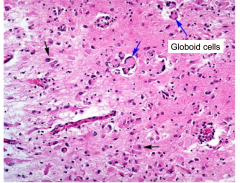
Globoid cells in Krabbe's Globoid Cell Leukodystrophy
|
|
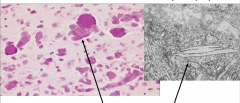
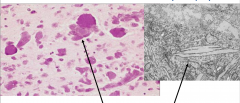
Characteristics of Krabbe's Globoid Cell Leukodystrophy
|
Loss of myelin
Accumulation of Globoid macrophages, cluster around vessels Severe astrocytosis Decreased numbers of oligodendrocytes EM - globoid cells contain crystalloid straight or tubular profiles |
|
|
Metachromatic Leukodystrophy
|
Lysosomal storage disease
autosomal recessive gene on chromosome 22 Deficiency of Aryl Sulfatase A |
|
|
Clinical Course of Metachromatic Leukodystrophy
|
Metachromatic lipids (sulfatides) accumulate in brain, peripheral nerves, and kidney
Sulfatide accumulation leads to breakdown of myelin, |
|
|
Diagnosis of Metachromatic Leukodystrophy
|
Demonstrate enzyme deficiency in urine, WBC, or fibroblasts
|
|
|
Clinical S/S of Metachromatic Leukodystrophy
|
Late Infantile (most common)
Intermediate Juvenile (each childhood type presents with gait disorder and motor symptoms with death in 5-10 years) |
|
|
Adult clinical S/S of Metachromatic Leukodystrophy
|
Usually present with psychosis and cognitive impairment, eventual motor symptoms
|
|
|
Treatment of Metachromatic Leukodystrophy
|
Bone marrow stem cell transplantation (before symptoms appear)
|
|

|
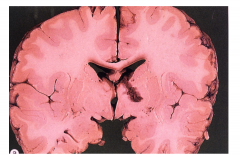
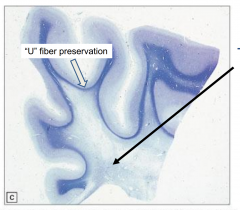
Metachromatic Leukodystrophy - the brain is externally normal and white matter is very firm
|
|
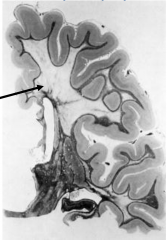
Characteristics of Metachromatic Leukodystrophy
|
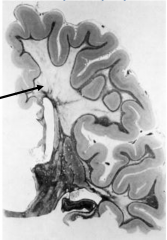
Marked loss of myelin
Preservation of "U" fibers (subcortical fibers) |
|
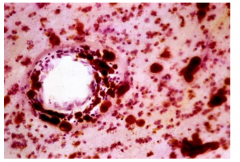
Characteristics of Metachromatic Leukodystrophy
|
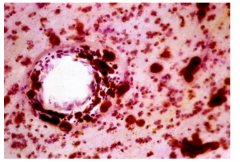
Metachromasia of white matter deposits (brown staining)
|
|
|
Adrenoleukodystrophy
|
Peroxisomal Disorder
- Peroxisomes - cytoplasmic spherical "microbodies" they contain catalase and are involved in fatty acid beta-oxidation - Decreased activity of very long fatty acyl-CoA synthetase (in peroxisomes) - Excess of very long fatty acid esters in plasma, cultured fibroblasts, and affected organs (CNS, PNS, adrenal glands) - X-linked (classic form) |
|
|
Clinical Signs of Adrenoleukodystrophy (classic form)
|
Classic Form:
- Onset 5-9 years or 11-21 years - Dementia, visual/hearing loss, seizures - Adrenal insufficiency follows Neuro S/S |
|
|
Clinical Signs of Adrenoleukodystrophy (Adrenomyeloneuropathy)
|
Occurs in adult (20-30)
Slowly progressive leg clumsiness/stiffness; eventual spastic paraplegia Adrenal insufficiency may precede Neuro S/S |
|

|
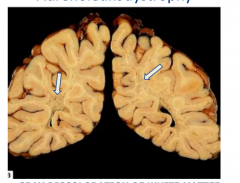
Adrenoleukodystrophy
- gray discoloration of white matter - marked firmness |
|

|
Adrenoleukodystrophy
-Severe demyelination |
|

|
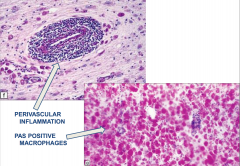
Adrenoleukodystrophy
- Perivascular inflammation - PAS positive macrophages |
|
|
Hepatic Encephalopathy
|
- Can occur as a complication of severe liver disease or chronic portocaval shunting.
- Pathogenesis is incompletely understood, but is partially related to hyperammonemia - Early manifestations include attentiveness and short term memory impairment - Later features include confusion, asterixis (flapping tremor of outstretched hands), drowsiness, stupor, and coma - Patients may also have foul breath (fetor hepaticus(, hyperventilation, gait disturbances, and choreoathetosis |
|
|
MRI Abnormalities of Hepatic Encephalopathy
|
Increased T1 signal in the globus pallidus, subthalamus, and midbrain, and cortical edema
|
|
|
Acute Hepatic Encephalopathy
|
Can be rapidly fatal
Chronic or repeated episodes can lead to permanent and progressive neuropsychiatric disturbances |
|
|
Treatment of chronic hepatic encephalopathy
|
Liver transplant
|
|

|
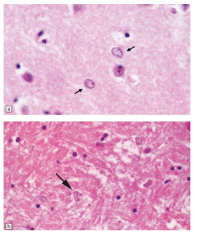
Hepatic Encephalopathy
Alzheimer type II astrocytes |
|
|
Hypoglycemia
|
Can be the result of insufficient food intake, through systemic diseases (hyperinsulinism, severe liver disease, or adrenal insufficiency) or through exposure to drugs that cause hypoglycemia (insulin)
|
|
|
How do patients with hypoglycemia typically present?
|
Headache
Confusion irritability incoordination Lethary that lead to stupor and coma |
|
|
MRI of hypoglycemia
|
Signal changes in the temporal, occipital, and insular cortices, hippocampus, and basal ganglia, often with thalamic sparing.
|
|

|
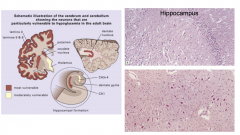
Hypoglycemia
- bottom picture is dentate nucleus |
|
|
Prolonged bouts of hypoglycemia can result in ?
|
Permanent brain damage
|
|
|
Treatment for hypoglycemia
|
Depends on the cause
- Restoration of glucose for exogenous causes - Removal of endogenous cases (liver, pancreatic, or adrenal tumors) |
|
|
Mitochondrial Diseases
|
Can cause a broad variety of clinical issues involving numerous organ systems
Brain and muscle involvement are common, but involvement of GI tract, heart, and/or peripheral nerves are also present |
|
|
Genetics of Mitochondrial Diseases
|
Multigenerational disease shows maternal inheritance
- Mitochondrial proteins are encoded within the mitochondrial and nuclear genome complicating the diagnosis - Diagnosis is based on a battery of clinical, imaging, pathological, and molecular assays. |
|
|
Leigh's Disease
|
- Mutation in nuclear DNA
- Cause enzyme deficiency in pathway converting pyruvate to ATP - Decreased activity of cytochrome C oxidase - Autosomal Recessive - Lactic acidemia |
|
|
Clinical S/S of Leigh's Disease
|
Arrest of devo
Hypotonia Seizures Extraocular palsies Death between 1 and 2 |
|
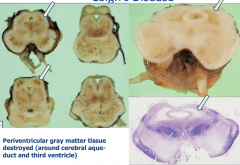
Leigh's Disease
|
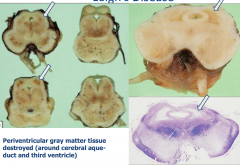
Periventricular gray matter tissue destroyed (around the cerebral aqueduct and third ventricle)
|
|
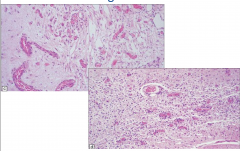
Leigh's Disease
|
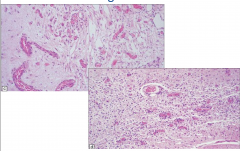
Spongiform appearance
Vascular Proliferation |
|
|
Thiamine (Vit B1 deficiency)
|
- Seen predominantly in chronic alcoholics
Can also be caused by starvation diets, hemodialysis, gastric stapling, |
|
|
Two Syndromes of Thiamine Deficiency
|
Wernike encephalopathy
Korsakoff Syndrome |
|
|
Wernike Encephalopathy - Clinical Presentation
|
Opthalmoplegia, Nystagmus
Ataxia Confusion, Disorientation, Eventual Coma |
|
|
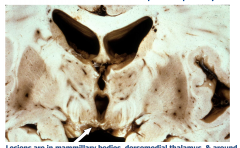
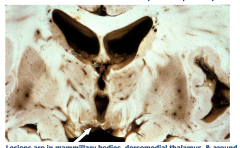
Wernicke Encephalopathy
Lesions are in the mammillary bodies, dorsomedial thalamus, and around the 3rd and 4th ventricles Acutely - gray-brown discoloration with petechial hemorrhages Chronic - Atrophy and discoloration of mammillary bodies |
|
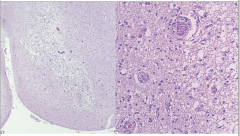
Pathology of Wernicke Encephalopathy
|
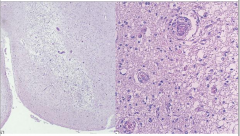
Pallor
Myelin Loss Prominent Vessels Macrophages Relative Preservation of Neurons |
|
|
Korsakoff Psychosis - Clinical S/S
|
Loss of anterograde episodic memory, confabulation, preserved intelligence and learned behavior
Hypothesized to result from repeated episodes of Wernicke's Encephalopathy No pathology distinct from Wernicke's Findings attributed to damage to medial dorsal nucleus of thalamus |
|
|
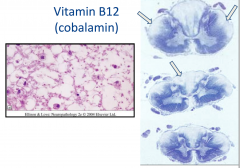
Vitamin B12 (cobalamin)
|
Subacute combined degeneration of spinal cord
Usually due to pernicious anemia |
|
|
Clinical Aspects of Vit B12 Cobalamin
|
Ataxia, Romberg, Spasticity, Decreased Reflexes, Mental Status Changes
|
|
|
Pathology of Vit B12 Cobalamin
|
- CNS and PNS involvement
- Spinal Cord: Anterior and Lateral corticospinal tracts and posterior columns are vacuolated and demyelinated - May have secondary axonal degeneration |
|
|

|
|
|
Carbon Monoxide
|
- Irreversibly binds to hemoglobin, displacing oxygen
- Binds to areas rich in iron (globus pallidus, substantia nigra) & causes necrosis - Degeneration of white matter also occurs - CO poisoning usually accompanied by hypotension/ischemia - Motor, cognitive, psychiatric, and parkinsonian signs and symptoms |
|
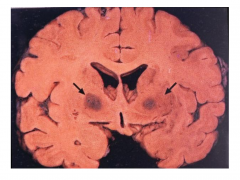
Acute Carbon Monoxide Poisoning
|
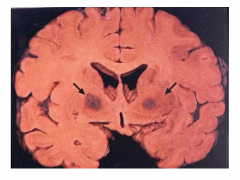
Acute Carbon Monoxide Poisoning
- Bilaterate pallidal cavitation |
|
|
Chronic Ethanol Toxicity (Alcoholism on the brain)
|
Clinically - truncal ataxia, nystagmus, and limb incoordination
Cerebellar degeneration - atrophy- especially of anterior superior vermis Dropout of Purkinje cells, internal granular cells, astrocytosis |
|

|
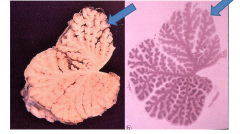
Chronic Ethanol Toxicity (Alcoholism on the brain)
Cerebellar degeneration - atrophy- especially of anterior superior vermis Dropout of Purkinje cells, internal granular cells, astrocytosis |
|
|
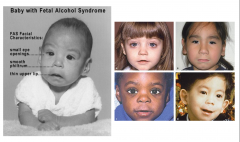
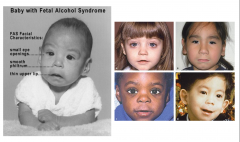
Fetal Alcohol Syndrome
|
Associated with low levels of alcohol consumption
|
|
|
Clinical Picture of Fetal Alcohol Syndrome
|
Growth Retardation
Facial Deformities - short palpebral fissure, epicanthal folds, thin upper lip, growth retardation of jaw - Cardiac defects - atrial septal defect - Delayed development and mental deficiency |
|
|
Pathological Findings of Fetal Alcohol Syndrome
|
- Microcephaly
- Cerebellar Dysgenesis - Heterotopic neurons - Hypothesized that acetaldehyde crosses placenta and damages fetal brain |
|
Fetal alcohol Syndrome
|
Fetal Alcohol Syndrome
|
|
|
Characteristics of Fetal Alcohol Sydrome
|
- Epicanthal Folds
- Flat Nasal Bridge - Small Palpebral Fissures - "Railroad Track" ears - Upturned Nose - Smooth Philtrum - Thin upper lip |
|
|
Methotrexate Toxicity
|
Intrathecal or Intraventricular Admin in combination with radiation may produce :
- disseminated necrotizing leukoencephalopathy - Particularly around the ventricles and deep white matter - Coagulative necrosis with axonal loss and mineralization |
|
|
Vincristine Toxicity
|
P.O. admin - sensory neuropathy
- Intrathecal admin - axonal swelling |
|
|
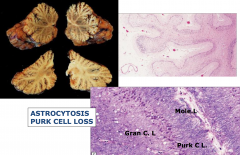
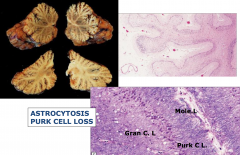
Phenytoin Toxicity
|
- Ataxia, nystagmus, slurred speech, and sensory neuropathy
- Atrophy of cerebellar vermis and loss of Purkinje cells and granule cells |
|
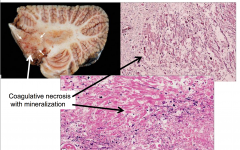
Methotrexate Toxicity
|
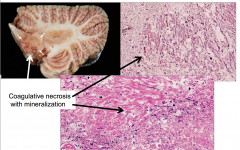
Methotrexate Toxicity
- Coagulative necrosis with mineralization |
|
Phenytoin Toxicity
|
Phenytoin Toxicity
|
|
|
Cocaine Toxicity
|
Seizures, strokes, hemorrhages
Infarcts and hemorrhages due to vasospasm, emboli, hypercoagulability, hypotension, drug contaminants |
|
|
Amphetamine Toxicity
|
Infarcts and hemorrhages
Attributed to vasculitis and hypertension |

