![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
30 Cards in this Set
- Front
- Back
|
A nucleus consist of what? |
- Protons - Neutrons |
|
|
What charge do protons have? |
Positive + |
|
|
What charge do neutrons have? |
No charge |
|
|
What charge do electrons have? |
Negative - 0 Mass |
|
|
Electron volt formular? |
- 1.6 x 10^-19 |
|
|
Atom Sumary |
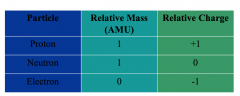
|
|
|
What are the four known forces in the universe? |
Strong Weak Electromagnetic Gravity |
|
|
Atomic or Proton Number (Z) |
atomic number (Z) = the no. of protons in the nucleus The single most important characteristic of an atom If an atom has a Z of 6, it is carbon Z of 92 corresponds to uranium |
|
|
Mass or Nucleon Number (A) |
mass number (A) = no. of protons + no. neutrons The number of neutrons in a nucleus affects the mass of the atom but not its chemical properties. |
|
|
Net charge = how protons and electrons balance out |
A neutral atom has no overall charge - so the charges of the protons and electrons are balanced For a neutral atom - Number of protons = Number of electrons |
|
|
Ionic Bonding (Gives Electrons) |
To become stable Sodium wants to LOSE 1 electron Chlorine want to gain 1 electronForms an ionic bond Each atom now an ion with net + and – chargeElectromagnetic force bonds the atoms together to form a moleculeSodium chloride |
|
|
Covalent Bond (Shared Electrons) |
If electrons are shared a covalent bond is formed |
|
|
Ions |
An atom that carries an electrical charge is called an Ion If the atom loses electrons, the atom becomes positively charged (+) If the atom gains electrons, the atom becomes negatively charged (-) |
|
|
Ions |
The number of protons does not change in an Ion. The number of neutrons does not change in an Ion. So, both the atomic number(Z) and the mass number (A) remain the same. |
|
|
Hydrogen Example |

|
|
|
Isotopes |
Isotopes are atoms of the same element having different masses, due to varying numbers of neutrons - same number of protons but different numbers of neutrons Atoms of the same element can have different numbers of neutrons. Thus, different mass numbers (A). |
|
|
Isotopes |
A nucleus with 6 protons and 6 neutrons will have the same chemical properties as a nucleus with 6 protons and 8 neutrons Although the two masses will be different. All chemical elements have many isotopes. |
|
|
Radioisotopes |
Atom with an unstable nucleus When nuclear force within nucleus can not overcome forces of repulsion, particles are ejected and / or radiation energy Radioactive decay Unstable nucleus because an unbalance between protons and neutrons. They change to a more stable form by the process of radioactive decay. |
|
|
Electron Volt (eV) |
The unit of energy used when discussing atomic structure is the electron volt.One electron volt is equal to the amount of energy gained by an electron travelling through an electrical potential difference of 1 V (Volt). 1 electron volt is equivalent to 1.60207 × 10 ^-19 J. Commonly expressed as keV (x 1,000) , MeV x 1,000,000) |
|
|
Electron Orbits and Energy Levels |
Electrons can only move and exist in specified shells around the nucleus Different shells represent different energy levels Known as orbits |
|
|
Electron Orbits and Energy Levels |
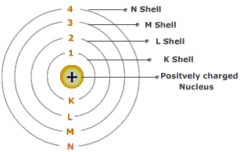
Different shells have different energy levels. Less electrons in outer shell makes it more reactive |
|
|
Electron Shells |
Number of electrons & their arrangement in shell determines how atom links with other atoms (i.e. chemical & physical properties) Bound by the electromagnetic force Inner shells occupied first Electrons in K are more strongly bound than L, M or N |
|
|
Electron Shells |
Electrons orbit around the nucleus in energy levels (shells).1st energy level K holds up to 2 electrons 2nd energy level L holds up to 8 electrons 3rd energy level M holds up to18 electrons 4th energy level N holds up to 32 electrons |
|
|
Electron Shells |
NOTEOutermost shell is know as the valance shell Contains a maximum of 8 electrons |
|
|
Valence Shell |
Outermost shell for any particular atoms is know as the valance shell Contains a maximum of 8 electrons Any atom that has a full valence shell are very stable very unlikely to react chemically In the periodic table the noble gases make a group of chemical elements with similar properties The six noble gases that occur naturally are helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and the radioactive radon (Rn). |
|
|
Pauli exclusion principle states that |
No two electrons in an atom can occupy exactly the same energy state Each electron shell must represent a cluster of energy levels rather than a single energy state |
|
|
Excitation and Ionisation |
Atom does not remain in this state for long Usually followed by electron rearrangement back to neutral (ground) state Emits energy in the form of a photon which has a specific frequency and wavelength |
|
|
Ionisation Energies - Oxygen |

|
|
|
Electron Energy States |
When electrons are given energy they will rise in energy level that the same amount of energy that was given. The more energy the electrons absorb the higher they rise Excited state |
|
|
Electron Energy States |
Electrons like to fall back down to their original state (ground state) As the electron falls back down to the ground state they release energy that caused them to rise in the first place The energy released is in the form of a photon - No mass - EM radiation (light) |

