![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
3 Cards in this Set
- Front
- Back
|
Alicyclic hydrocarbon
How to pronounce : http://www.howjsay.com/index.php?word=alicyclic&submit=Submit |
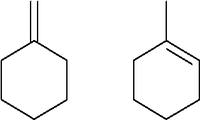
Alicyclic is a compound that contains one or more rings of carbon either saturated or unsaturated and can have other chains attached.
Examples of these compounds are cycloalkanes and cycloalkenes such as cyclobutane. |
|
|
Aliphatic hydrocarbon
How to pronounce : http://www.howjsay.com/index.php?word=aliphatic&submit=Submit |
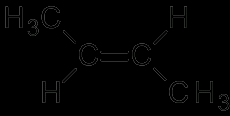
Aliphatic hydrocarbons are compounds composed of carbon and hydrogen atoms and can be in many different forms. Such as saturated or unsaturated.
These are any hydrocarbons that do not contain other elements. Examples of these are butane, pentane, butene etc. |
|
|
Lone pair
|
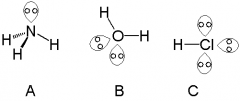
A lone pair is a pair of electrons that are not part of the bonding between the atoms in the molecule. These are found normally on the outer shell and are responsible for another type of bonding.
Lone pairs can be found with in the atoms in nitrogen groups such as ammonia. Examples are Ammonia and water. |

