![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
24 Cards in this Set
- Front
- Back
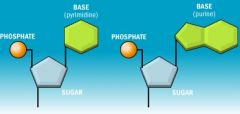
What type of structure does this image depict? |
Nucleotide
|
|
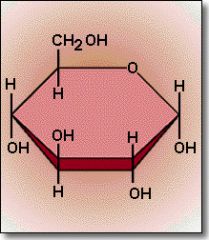
What is his an example of?
|
Carbohydrate ( monosaccharide )
|
|
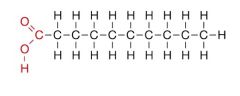
What type of lipid is this and name some characteristics?
|
Saturated fat: from animal fat, solid at room temperature, single carbon bond
|
|
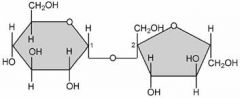
What is this an example of?
|
Polysaccharide (carbohydrates)
|
|
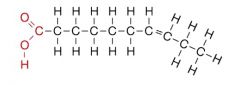
What type of lipid is this and name it's characteristics? |
Unsaturated fat: double bonded at carbon, liquid at room temperature, comes from a plant |
|
|
Which of the pHs listed below represents the strongest base?
7 10 13 15 |
13
|
|
|
Covalent bonds result from what?
the sharing of equal numbers of electrons by two atoms the exchange of equal numbers of electrons by two atoms the combination of two atoms of the same valence sharing of unequal numbers of electrons by two atoms |
the sharing of equal numbers of electrons by two atoms
|
|
|
An element having 8 protons, 8 neutrons, and 8 electrons would weigh _____ daltons.
8 32 24 16 |
16
|
|
|
The atomic number is equal to the number of what in an atom? |
protons
|
|
|
Which of the following pH values represents the greatest concentration of H+ ions?
|
3. 2 |
|
|
Of the following elements, which is the least common in living organisms?
Sodium Oxygen Hydrogen Nitrogen Carbon |
Sodium |
|
|
The chemical properties of an atom are primarily determined by the number of what?
neutrons it has in its nucleus isotopes it forms protons it has in its nucleus energy levels it has electrons it has in its outermost energy level |
electrons it has in its outermost energy level
|
|
|
What are the top 4 elements found in humans?
|
Carbon , hydrogen, oxygen, nitrogen
|
|
|
How many electrons are located in the valance shell of oxygen?
|
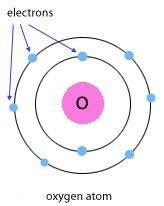
6
|
|
|
What are the difference between a compound and molecule?
|
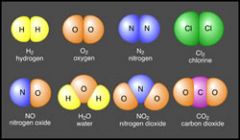
A molecule is two or more atoms chemically bonded
A compound is a molecule containing two or more different atoms (See image above) |
|
|
What is a molecular weight of a substance expresses in grams?
|
Mole
|
|
|
Hydrophobic interactions are exhibited by what?
|
Nonpolar molecules
|
|
|
What do molecules lose during oxidation?
|
They lose electrons
|
|
|
Which chemical bond is the strongest?
A. Ionic bond B. single covalent bond C. Double covalent bond D. Hydrogen bond |
C. Double covalent bond
|
|
|
Functional Group
|
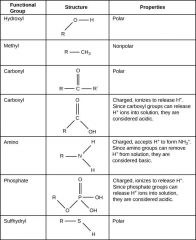
Group of atoms that provides or imparts a specific function to a carbon skeleton
|
|
|
Structural Isomers
|
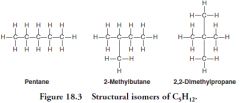
Molecules that share a chemical formula but differ in the placement of their chemical bonds
|
|
|
pH Scale
|
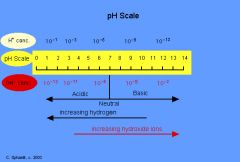
Measures the concentration of hydrogen ions (H+) in a solution
|
|
|
What are the properties of a phospholipid?
|
They are amphipathic which means they have a region that is hydrophobic and a region that is hydrophilic.
They form the bilayer of all mbira be found in and around cells Polar head(hydrophilic) and non polar tail(hydrophobic). |
|
|
Neutral fats (triglycerides)
|
Three fatty acids chains attach to a single glycerol molecule by dehydration synthesis. Neutral fats store energy fuel, insulate body tissues, and cushions and protects organs
|

