![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
87 Cards in this Set
- Front
- Back
|
Define PH
|
pH is a mathematical expression:
pH = -log10 [H+] |
|
|
log 10 is a question asking:
|
what is the power to which 10 is raised to give the number following the log10 expression?
|
|
|
[H+] is:
|
the hydrogen ion concentration in moles/Liter
|
|
|
log10 10(3) is a question. What is it asking:
|
What is the power to which 10 is being raised?
Examples: log10 |
|
|
log10 1000 =
|
3 because 1000 = 103
|
|
|
log10 100 =
|
2 because 100 = 102
|
|
|
log10 10 =
|
1 because 10 = 101
|
|
|
log10 0.001 =
|
-3 because 0.001 = 10-3
|
|
|
log10 0.1 =
|
-1 because 0.1 = 10-1
|
|
|
log10 1 =
|
0 because 1 = 100
|
|
|
Try these:
log10 10,000 = log10 100,000 = log10 0.0001 = log10 0.000001 = |
1. 4
2. 5 3. -4 4. -6 |
|
|
For our purposes, we will stick to whole number powers of 10 so that we do not need a calculator with a log function. For example, log10 123 = 2.0899. Explain
|
Because 123 is between 100 and 1000 we know its log10 must between 2 (which is the log10 of 100) and 3 (which is the log10 of 1000).
|
|
|
If a solution has a hydrogen ion concentration of 10-4 moles/L, what is its pH?
|
[H+] = 10-4 moles/L
pH = -log10 [H+] pH = -log10 [10-4] pH = -(-4) pH = 4 |
|
|
A solution with:
[H+] = 10-2 moles/L will have a pH of |
2
|
|
|
A solution with:
[H+] = 10-5 moles/L will have a pH of |
5
|
|
|
A solution with:
[H+] = 10-9 moles/L will have a pH of |
9
|
|
|
[H+] ↑ =
|
pH ↓
|
|
|
The concept of pH comes from:
|
the ionization of water into hydrogen and hydroxyl ions
|
|
|
Ionization is
|
is the process of giving up or accepting negatively charged electrons.
|
|
|
Ionization of water:
|
H2O ↔ H+ + OH
|
|
|
Kw is defined as:
|
the ion product constant (or ion product for water).
|
|
|
The ion product is expressed:
|
Kw = [H+][OH-]/H20
|
|
|
Although the relative concentrations of hydrogen and hydroxyl ions may vary greatly, their product always
|
remains constant.
|
|
|
The value of Kw has been determined to be 1 X 10-14 at 25C. The concentrations of hydrogen and hydroxyl ions are equal since ionization of one molecule of water would produce one of each ion.
Therefore, for distilled water at 25C: |
[H+] = [OH-] and
[H+] x [OH-] = 1 X 10-14 10-7 x 10-7 = 10-14 [H+] = 1 X 10-7 moles/L [OH-] = 1 X 10-7 moles/L |
|
|
The pH of Blood is
|
7.4
|
|
|
A one unit change in pH represents a:
|
a 10-fold change in [H+].
|
|
|
The pH scale goes from 0 to 14 because
|
[H+] does not exceed 1 X 100 or 1 mole/L nor goes below 1 X 10-14 moles/L in an aqueous solution.
|
|
|
pH 7 is neutral because
|
[H+] = [OH-].
|
|
|
An acid is
|
a substance that produces H+ in solution.
|
|
|
A base is
|
a substance that accepts H+, but it is not necessarily an OH-.
|
|
|
Almost all bases are
|
negatively charged (Cl-, HCO3-, etc).
|
|
|
Some proteins with negatively
charged areas can act as |
bases
|
|
|
hemoglobin is a coiled protein with negative areas. These areas can attract:
|
H+ and act as bases.
|
|
|
If pH is below 7
|
the solution is acidic because [H+] > [OH-] or [total of all base contributors]
|
|
|
If pH is above 7
|
the solution is basic or alkaline because [H+] < [OH-] or [total of all base contributors].
|
|
|
A buffer is
|
a solution that resists changes in pH when an acid or base is added to it. It can do this in two ways: 1) it can give up H+ or 2) accept H+.
|
|
|
Define Maximum buffering capacity
|
you can exceed a solution’s ability to resist changes in pH. There is a total quantity of an acid or a base that a given buffer can “neutralize” either by donating or accepting H+.
|
|
|
Name Important Buffering Systems:
|
Blood: 1) hemoglobin, 2) plasma proteins, and 3) carbonic acid-bicarbonate ion
Urine: 1) phosphate buffers, 2) NaH2PO4 |
|
|
Enzymes
|
increase the amount of product produced per time.
|
|
|
Most enzymes are
|
proteins (amino acids in a chain).
|
|
|
Proteins depend on
|
structure to work properly.
|
|
|
Two important factors can change protein structure:
|
pH and temperature.
|
|
|
In the stomach, enzymes work best at a pH of
|
2.5
|
|
|
In the small intestines, enzymes work best at a pH of
|
7.1
|
|
|
IN order for the lungs to inflate and deflate the volume of the thoracic cavity must
|
Increase and decrease
|
|
|
Inflation and deflation of the lungs is accomplished using which muscles
|
intercostal muscles, the diaphragm and often the abdominal muscles
|
|
|
At rest the contraction of the External Intercostal muscles accounts for about
|
66% of the increase in the size of the thoracic cavity
|
|
|
What percentage of contraction is accounted for by diaphragm contraction?
|
33%
|
|
|
Why is it important that the pH of body fluid be maintained within narrow limits.
|
Assuming that the enzymes are operating at the optimum pH, increases or decreases in pH will reduce the enzyme activity. If this is a cellular enzyme, then cellular metabolism will be adversely affected.
|
|
|
Function of buffering systems:
|
To resist changes in pH
Give up (produce) hydrogen ions and also bind hydrogen ions. |
|
|
Buffer systems may also consist of a single molecule (e.g. protein) that can produce
|
ions or accept hydrogen ions.
|
|
|
Buffer systems in living organisms or their environment are
|
aqueous solutions of the buffer
|
|
|
Buffer systems in the body exist what type of medium:
|
blood, interstitial fluid and urine, etc.
|
|
|
Buffer systems consist of a weak acid and the completely ionized salt of its conjugate base. The weak acid can
|
Produce ions and the conjugate base can bind with hydrogen ions.
|
|
|
Name buffer systems of plasma
|
bicaronate - most important
plasma proteins phosphates |
|
|
Name buffer systems of RBC
|
hemoglobin
phosphates bicarbonate |
|
|
1. trachea
2. tracheal cartilages 3. right lung 4. left lung 5. pleural cavity 6. diaphragm 7. heart 8. mediastinum 9. liver, left lateral lobe 10. liver, right/left medial lobes 11. gall bladder 12. right phrenic nerve |
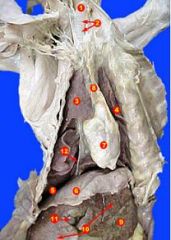
|
|
|
1. Thyrohyoid Membrane
2. Thyroid Cartilage 3. Cricoid Cartilage 4. Esophagus (behind trachea) 5. Trachea 6. Larynx 7. Tracheal Rings |
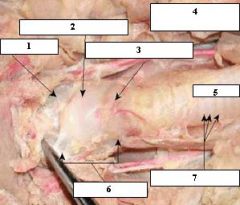
|
|
|
What is a spirometer?
|
an instrument used to measure lung volumes and capacity
|
|
|
what is a spirogram?
|
a graph used to measure the spirometer's inspiratory and expiratory volumes
|
|
|
define TV?
|
Tidal Volume - the volume of air moved into and out of the lung during normal breathing (about 500 ml)
|
|
|
define IRV
|
Inspiratory Reserve Volume - the volume of air that can be forcibly inhaled after normal breathing (about 3100 ml)
|
|
|
define ERV
|
Expiratory Reserve Volume - the volume of air that can be forcibly exhaled after normal breathing (about 1200 ml)
|
|
|
define RV
|
Residual Volume - the volume of air that remains in the lungs after maximum exhalation (about 1200 ml)
|
|
|
how many times per minute do normal individuals breath?
|
12 times per minute
|
|
|
define inspiratory capacity
|
TV + IRV = about 3600 ml
|
|
|
define functional residual capacity
|
ERV + RV = about 2400 ml
|
|
|
define vital capacity
|
TV + IRV + ERV = about 4800 ml
|
|
|
define total lung capacity
|
TV + IRV + ERV + RV = approximately 6000 ml
|
|
|
What does a spirogram monitor?
|
air flow within the respiratory system
|
|
|
How is normal inspiration initiated?
|
when impulses from the inspiratory region stimulate inspiratory muscles, the diaphragm and the external intercostal to contract. contraction of the muscles moves air into the lungs
|
|
|
what happens during the normal tidal cycle which leads to exhalation?
|
for the next 3 seconds inspiratory muscles are not stimulated so passive elastic recoil produces exhalation and causes air to move out of the lungs
|
|
|
what stimulates the inspiratory muscles
|
after 3 seconds of relaxation the inspiratory area stimulates the inspiratory muscles, diaphragm and external intercostals.
|
|
|
What does the inspiratory area control?
|
Normal ventilation while expiration is passive.
|
|
|
what happens during forced breathing?
|
the inspiratory area stimulates accessory inspiratory muscles and inspiration is more forceful.
|
|
|
what happens during normal breathing?
|
nerve impulses are automatically generated from the inspiratory area in the medulla
|
|
|
the lung volume or capacity that is equal to TV + IRV + ERV + RV
|
total lung capacity
|
|
|
the lung volume or capacity that is equal to IC - IRV =
|
tidal volume
|
|
|
the lung volume or capacity that is equal to FRC - ERV =
|
residual volume
|
|
|
the volume of air remaining in the lungs after normal expiration
|
functional residual capacity
|
|
|
lung volumes are measured
|
to examine if pulmonary functions are improving
for diagnosis of respiratory disease to observe if pulmonary functions are deteriorating |
|
|
the lung volume or capacity that is equal to IC - TV =
|
inspiratory reserve volume
|
|
|
If you breathe in as deeply as you can then exhale as deeply as possible which lung capacity have you demonstrated?
|
vital capacity
|
|
|
the lung volume or capacity that is equal to TV + IRV =
|
inspiratory capacity
|
|
|
what would be considered a dependent variable during a lung capacity experiment?
sex age and height level of physical activity respiratory volume |
respiratory volume
|
|
|
what would be considered an independent variable during a lung capacity experiment?
sex age and height level of physical activity respiratory volume |
level of physical activity
|
|
|
what would be considered a controlled variables during a lung capacity experiment?
sex age height respiratory volume |
sex, age and height
|

