![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
175 Cards in this Set
- Front
- Back
|
Name the different follicles through the entire maturation process of a menstrual cycle.
|
Primordial follicle -> primary follicle -> secondary follicle -> Graafian follicle
|
|
|
What determines the follicle that becomes the dominant follicle during each menstrual cycle?
|
The dominant follicle is the one that develops LH receptors on its granulosa cells
|
|
|
During the follicular phase of the ovarian cycle, which hormones do each of the follicular cell types produce?
|
Theca interna cells - androstenedione
Granulosa cells - estrogen Note that theca externa cells do not have an endocrine function. |
|
|
At puberty, how many follicles remain in a female?
|
400,000
|
|
|
How many times does a woman ovulate in her lifetime?
|
500
|
|
|
During follicular phase of ovarian cycle, which hormone promotes theca cells to produce androstenedione? granulosa cells to produce estrogen?
|
LH promotes theca cells to make androstenedione.
FSH promotes granulosa cells to make estrogen. |
|
|
What does estrogen promote during the follicular phase?
|
Granulosa cell proliferation
Estrogen receptors FSH receptors LH receptors (on granulosa cells of dominant follicle) |
|
|
How many hours after LH surge starts before ovulation occurs?
|
It takes about 36-40 hours
|
|
|
List the mechanisms by which LH surge causes ovulation
|
1. Neutralizes the action of oocyte maturation inhibitor
2. Induces prostaglandin endoperoxidase synthase, which results in prostaglandins, thromboxane, and leukotriene production. Prostaglandins and leukotrienes cause contraction of follicular wall. |
|
|
What are the three phases of endometrial cycle?
|
1. Proliferative phase (estrogen causes proliferation of uterus lining)
2. Secretory phase (progesterone initiates secretion of acid mucin) 3. Atretic phase (progesterone decreases, uterine lining sloughs) |
|
|
What are the contraceptive options in the following categories?
- Most effective - Very effective - Moderately effective - Effective |
Most effective
- male/female sterilization - IUD, IUS Very effective - Pills - Injectables - Patch - Ring Moderately effective - Condom Effective - Fertility awareness - Cervical cap |
|
|
What is the standard way of comparing effectiveness of a contraceptive method?
|
Pearl index - give contraceptive to 100 women, and assuming typical use, measure % of them that get pregnant after 1 year.
|
|
|
How many months after a vasectomy does a male become infertile?
|
It takes 3 months to achieve azoospermia.
|
|
|
What is the MOA of estrogen and progestin in combined oral contraceptives?
|
Progestin is main factor in preventing pregnancy
□ Suppresses secretion of gonadotropin (mostly LH) from pituitary gland Estrogen □ Inhibits FSH secretion □ Also works synergistically with progestin to affect uterine lining and cervical mucus production |
|
|
What is the MOA of progestin-only contraceptives?
|
-Thickening cervical mucus
-Causing endometrial atrophy -Reduce activity of cilia in fallopian tubes |
|
|
What is the MOA of depo-provera (depot medroxyprogesterone acetate)?
|
-Thickening cervical mucus
-Causing endometrial atrophy -Reduces activity of cilia in fallopian tubes -Inhibits ovulation |
|
|
What is Plan B? How do you administer Plan B? What is the MOA?
|
It is progestin only.
2 doses at the same time, within 72 hours after intercourse. MOA - May inhibit or delay ovulation - Create deficient luteal phase - May affect tubal transport of sperm or ova |
|
|
What is the decidual reaction?
|
During secretory phase, endometrium undergoes changes to prepare for implantation.
- estrogen and progesterone are secreted by the corpus luteum, and cause endometrium to thicken, glands to form, and increased vascularization. - endometrial cell size increases, cell shape changes - formation of intercellular junctions - development of intracellular organelles for protein synthesis and secretion |
|
|
Which day (after conception) is the morula stage?
blastocyst stage? |
4 days and 5 days respectively
|
|
|
When is the window of implantation, relative to the time of fertilization?
|
6-7 days after fertilization
|
|
|
Describe the three stages of implantation.
|
1. Apposition - microvilli on trophoblast interact with pinopodes of endometrium
2. Adhesion - cell adhesion molecules interact 3. Invasion - blastocyst penetrates uterine epithelium |
|
|
What is the decidua basalis?
|
Part of the endometrium during pregnancy - the site of implantation, where the maternal portion of the placenta is
|
|
|
What does the term "hemochorial placentation" mean?
|
The maternal blood is in direct contact with trophoblast cells.
The barrier between maternal blood and fetal blood is of fetal origin. This includes: - trophoblast - fetal connective tissue - fetal endothelium |
|
|
When does placentation begin (relative to date of conception)?
When do lacunar spaces form? When does the basal plate form? |
Day 6-7, right after implantation
Day 9-12 Day 22 |
|
|
When does maternal placental blood flow begin?
|
10 weeks gestation
|
|
|
What are the three fates of the cytotrophoblast cells?
|
1. Fusion to form syncytiotrophoblast cells
2. Continued proliferation within the villi 3. Extravillous trophoblast, which invades into spiral arteries and transforms them into wide, flaccid non-reactive vessels which can accommodate massive placental blood flow (there are two waves, one at 8 weeks, one at 14 weeks). Failure of this process can cause pre-eclampsia |
|
|
What is the difference between primary, secondary and tertiary chorionic villi?
|
• Day 12 - primary villi - inner villous cytotrophoblast, outer syncytiotrophoblast
• Day 15 - secondary villi - mesenchymal intrusion, derived from extraembryonic mesoderm • Day 17 - tertiary villi - fetal capillary networks develop within the villus mesenchyme |
|
|
What are the different branches of the chorionic villi called, as they get smaller and smaller?
|
Stem villi
Intermediate villi Terminal villi |
|
|
What disappears - cytotrophoblast or syncytiotrophoblast?
|
Cytotrophoblast
|
|
|
Name the arteries that bring blood to the uterus, down to the arteries that will go into the intervillous spaces of the placenta.
|
Uterine artery
Arcuate arteries Radial arteries Spiral arteries (supply functional layer) and straight arteries (supply basal layer) |
|
|
What is the function of the yolk sac?
|
It functions as a hematopoietic organ
|
|
|
What comprises the chorion?
|
Cytotrophoblast + extraembryonic mesoderm
|
|
|
How much of the oxygen and glucose supplied to the uterus does the placenta use?
|
1/2 of the oxygen
2/3 of the glucose |
|
|
How does fetus live with low arterial pO2 (usually 30 mmHg)?
|
Despite the low pO₂, fetal blood can carry the same quantity of oxygen to tissues as mother, because:
1. HbF oxygen dissociation curve is shifted to the left 2. Fetal Hb concentration is higher 3. Fetal CO2 transfer to mother leads to double Bohr effect 4. Higher cardiac output |
|
|
Which nutrients/molecules are transported by the following methods:
passive diffusion facilitated diffusion active transport endocytosis |
• Passive diffusion
O₂ and CO₂ Ketones Some FA Electrolytes: Na+, K+, Cl- Water • Facilitated diffusion Glucose Lactate • Active transport Amino acids (except glutamate and aspartate) Ca²⁺, PO₄²⁺, Mg Ascorbic acid • Pinocytosis/endocytosis LDL IgG |
|
|
In what forms are non-protein nitrogens excreted via the placenta?
|
Urea, uric acid, and creatinine.
The diffusion of urea is faster than creatinine. |
|
|
What drugs are known to cross placenta and affect baby?
|
Anesthetic gases
Propylthiouracil They are liposoluble |
|
|
What is the role of progesterone during pregnancy?
|
□ Substrate for fetal adrenal gland production of gluco and mineralocorticocoids
□ Immunosuppressive role □ Role in implantation, and maintenance of pregnancy and parturition □ Metabolites contribute to pregnancy refractory state to angiotensin II □ Smooth muscle relaxation and vasodilation □ Possible role in increased ventilation |
|
|
What are the site(s) of production of progesterone in the maternal-placental-fetal unit?
|
Before 10 weeks - corpus luteum
After 10 weeks - placenta Also the decidua and fetal membranes |
|
|
What is the role of estrogen during pregnancy?
|
□ Increase uterine blood flow and cardiac output via peripheral vasodilation
□ Uterine muscle growth (hyperplasia and hypertrophy) □ Parturition (contractions) □ Preparation for lactation □ Increase liver production of ® Binding globulins (TBG, CBG) ® Renin substrate ® Angiotensinogen □ Inhibits maternal and fetal adrenal 3β-OH dehydrogenase (responsible for converting DHEA to androstenedione). This favours excretion of DHEA □ Increases insulin response to glucose |
|
|
What is the site of production of estrogens in the maternal-placental-fetal unit?
|
Placenta
|
|
|
What is the site of production of corticosteroids in the maternal-placental-fetal unit?
|
Fetal adrenal glands
|
|
|
What is the role of corticosteroids in fetus?
|
Fetal preparation for extrauterine life
- Promotes lung maturation - Increases fetal glycogen storage in preparation for birth - Maturation of liver enzymatic systems Physiologic changes of pregnancy - Maternal fluid expansion |
|
|
When can a pregnancy test first become positive, relative to date of missed period?
|
2-4 days before date of missed period
|
|
|
What hypothalamic-pituitary hormones are produced by the syncytiotrophoblast and cytotrophoblast?
|
Cytotrophoblast acts like hypothalamus, and syncytiotrophoblast acts like the pituitary.
Syncytiotrophoblast □ Human chorionic thyrotropin (like TSH) □ Human chorionic adrenocorticotropin hormone (like ACTH) Cytotrophoblast □ TRH □ CRH □ GnRH □ Inhibin |
|
|
What is the role of human placental lactogen (HPL)?
|
□ Lactogenic activity
□ Supports nutrition needs of fetus via: ® Stimulates lipolysis in fasting state ® Diabetogenic effect in fed state |
|
|
What is the role of relaxin?
|
□ Maintenance in early pregnancy
□ Cervical softening in early labour |
|
|
What is the site of production of relaxin in the maternal-placental-fetal unit?
|
Corpus luteum
Placenta Decidua Chorion |
|
|
What is the role of the amniotic fluid?
|
1. Fluid filled compartment
• Supplies EGF-like growth factors and prolactin, which help lung and GI development • Allows movements of fetus, preventing contractures • Protection against umbilical cord compression • Protection against trauma 2. Provides fetal extracellular fluid site 3. Provides temperature homeostasis 4. Prevents infection via antibacterial properties 5. Acts as communication system between fetus and mother |
|
|
What is the volume of amniotic fluid by week 24?
|
At 24 weeks, 700 cc
|
|
|
Effects of PGE₂, relaxin, and PGF₂α, endothelin-1
|
PGE₂ - cervical ripening
Relaxin - cervical ripening PGF₂α - contraction Endothelin-1 - involution of uterus after delivery of placenta |
|
|
What are Leopold's manoeuvres?
|
First manoeuvre - palpate the uterine fundus for contents
Second manoeuvre - palpate the fetal back on one side, and limbs on other Third manoeuvre - palpate the fetal presenting part just above the symphysis Fourth manoeuvre - Facing the feet, determine the fetal position (also confirmed on internal exam with a dilated cervix) |
|
|
Breakdown of the phase 2 of labour into further stages.
|
Stage 1 (of phase 2): dilation of cervix
- Latent phase (3-4 cm) - Active phase (rate of dilation increases. Rate is 1 cm/hour for nullipara, 1.2 cm/hour for multipara). Stage 2 - delivery of infant. 50 min - 3 hours for nullipara. 20 min for multipara. Stage 3 - delivery of placenta and membranes. 15-30 min Stage 4 - stabilization of maternal condition, bonding. 1-1.5 hours |
|
|
What are the rates of cervical dilation during the active phase of labour?
|
Nulliparas - 1.0 cm/hour
Multiparas - 1.5 cm/hour |
|
|
What are the fetus' cardinal movements of labour during phase 2, stage 2 of labour?
|
1. Engagement - biparietal diameter of the fetal head passes through the pelvic inlet
2. Descent - initiated with the start of stage 2 3. Flexion - as descending head meets resistance of the pelvic floor, it passively flexes 4. Internal rotation - occiput rotates to come under the symphysis in direct anterior-posterior direction 5. Extension - occiput comes into direct contact with the inferior part of the maternal symphysis and swivels under the bone, extending its head as it comes clear. The chin delivers last 6. Restitution/external rotation - head returns to its original left occiput anterior oblique position, and immediately after, continues the process of rotation to a left occiput transverse position in order to bring the fetal shoulders in line with the anterioposterior diameter of the pelvic output 7. Expulsion - occurs as the anterior shoulder comes under the symphysis, followed shortly thereafter by the posterior shoulder distending the perineum |
|
|
How long does the phase 2, stage 2 of labour last in nulliparas women? multiparas women?
|
Nulliparas: 50 min - 3 hours
Multiparas: 20 min |
|
|
What are the classic signs of placental separation?
|
□ Gush of blood
□ Uterus becomes firm and globular □ Lengthening of cord □ Fundus rises up |
|
|
Which spinal segments do pain afferents travel via during...
- first stage of labour? - end of first stage, and second stage? |
During most of first stage of labour:
• T10-L1 During later part of first stage, and second stage of labour: • L1-S4 |
|
|
For hCG, TSH, LH and FSH, which part of the molecule is the unique part?
|
The β subunit
|
|
|
What tests are done at the first prenatal visit of a healthy pregnant woman?
|
• Confirm pregnancy with in-office urine test
• Urine analysis (for protein and glucose) • Hb and hematocrit • Rh factor • Blood type • Pap smear (if not done in the past 6 months) |
|
|
When can fetal heart sounds can be seen on ultrasound?
|
6 weeks GA by transvaginal US
8 weeks GA by transabdominal US |
|
|
Which part of the fallopian tube is the best for fertilization to occur?
|
Ampulla.
(Order from fibriae into uterus is: infundibulum, ampulla, isthmus, intramural part) |
|
|
At what developmental week of the fetus do primordial germ cells migrate from yolk sac into gonadal primordia?
|
6th week of embryonic development
|
|
|
Name the germ cell as it undergoes spermatogenesis. How long does the process take?
|
• Spermatogonia (diploid 2N)
• →1° spermatocyte (diploid 4N, arrested in M1) • →2° spermatocyte (haploid 2N, MII, seldom seen because they immediately divide to form spermatids) • →spermatid (haploid N) • →spermatozoa (haploid N, maturation of spermatids via spermiogenesis) Takes 64-74 days |
|
|
What is spermiogenesis?
What are the four stages of spermiogenesis? |
Maturation of spermatid into spermatozoa (no division involved). Occurs while the spermatid head is embedded in Sertoli cell.
GCAM 1. Golgi stage (acrosome and centrioles develop) 2. Cap stage (polarization) 3. Acrosome 4. Maturation (pinch off excess cytoplasm, elongation, release) |
|
|
Function of Sertoli cells vs Leydig cells.
|
Sertoli:
- needed for spermiogenesis process (spermatid maturation into spermatozoa) - produce Androgen Binding Protein (needed to maintain high local levels of T) - maintain blood-testis-barrier Leydig cells - produce testosterone |
|
|
What is the function of the prostate? What is in its secretions?
|
Secretes PSA, which liquefies fibrin clots during ejaculation.
Secretion also contains acid phosphatase, citric acid, zinc. |
|
|
What is inhibin?
|
In females, it is a hormone secreted by granulosa cells that inhibits release of FSH from pituitary.
In males, it is secreted by Sertoli cells, and also inhibits release of FSH from pituitary. |
|
|
What is the MOA of a copper IUD?
|
Copper ions inhibit sperm motility
Sterile inflammatory reaction created in endometrium phagocytoses the sperm |
|
|
How long postpartum is it contraindicated for a woman to use combined oral contraceptives?
|
Before 3 weeks if not breastfeeding.
Before 6 weeks if breastfeeding, because this is how long it takes for lactation to be well established. |
|
|
What cancers are increased by using oral contraceptives?
What cancers are reduced? |
Increased risk of
- cervix adenocarcinoma - hepatocellular adenoma Reduced risk of - breast benign disease - endometrial cancer - ovarian cancer and physiologic cysts |
|
|
How long does does NuvaRing (etonorgestrel and EE) work for?
|
It is placed in vagina for 21 days, discarded, and then a new one inserted after 7 days.
|
|
|
How long does Evra, a transdermal patch containing norelgestromin and EE, work for?
|
Apply once per week for 3 weeks, then 1 week off.
|
|
|
What is Micronor?
How is it used? |
Progestin-only OC.
- norethindrone - must take at the same time everyday, with only a 2 hour window |
|
|
How often Depo Provera be injected to be effective at contraception?
What are a few significant disadvantages? |
Every 3 months.
Bone density decrease. However, this can be reversed when depo is stopped (ever after 4 years of use). Hypoestrogenism, causing dyspareunia, hot flashes, decreased libido. Not immediately reversible. |
|
|
How effective is "lactational amenorrhea method"?
|
If the mother is exclusively breastfeeding day and night, then the failure rate within the first 6 months is 0.5-2%
|
|
|
How soon after coitus does emergency contraceptive method need to be employed to be effective?
|
Copper IUD - within 5 days (probably 7 days is still ok)
Plan B - within 3 days (probably 5 days is still ok) |
|
|
After emergency contraceptive is given, will next menses be early, on-time, or late?
If there is no bleeding for __ days after the emergency contraceptive is given, woman must get pregnancy test. |
It can be early, on-time, or late.
21 days. |
|
|
Define infertility.
Primary infertility? Secondary infertility? |
The inability to conceive or carry to term a pregnancy after one year of regular, unprotected intercourse
Suggests ↓ capacity to conceive and reproduce. It does not mean that it is irreversible. Primary infertility - couple who has never achieved a pregnancy. Secondary infertility - at least one previous conception has taken place. |
|
|
What is fecundability?
|
Probability of conceiving during 1 monthly cycle.
|
|
|
What are the female causes of infertility?
|
Ovulatory dysfunction
-Hypothalamic hypogonadism -Pituitary (prolactinoma, deficiency in LH/FSH) -PCOS -Premature ovarian failure -Systemic disease (thyroid, Cushing, chronic renal/hepatic failure) -Stress, nutrition, excessive exercise Outflow tract (tubal, uterine, cervical problems) -PID -Fibroids -Asherman syndrome (intrauterine adhesions) -Uterine anomalies -Anti-sperm antibodies Endometriosis |
|
|
What is the % of women that can conceive within 1 year of frequent trying, between the ages of 25-29?
|
78%
|
|
|
Based on age, when should you refer or initiate infertility evaluation?
|
< 35 years of age, begin eval after 12 months of trying to conceive.
35-40 years of age, begin eval after 6 months. 40 years - consider immediate eval. |
|
|
If a woman who is being evaluated for infertility is anovulatory, which things must you look for during history and physical exam?
|
Thyroid problem
Hirsuitism Galactorrhea |
|
|
Sign/symptoms of endometriosis.
|
Dysmenorrhea
Deep dyspareunia Pre-menstrual spotting Infertility Painful nodules felt in posterior cul-de-sac on rectovaginal exam Bowel and bladder symptoms - dysuria, hematuria, constipation, diarrhea, hematochezia |
|
|
List main investigation tests for infertility.
|
Assess ovulation
1. Spinnbarkeit (cervical mucus) 2. Basal body temperature chart 3. Serum progesterone measured 21-22 days after last onset of menses 4. Urinary LH test, done 24 hours before expected ovulation time 5. Day 3 FSH, to assess ovarian reserve (if high, could indicate ovarian failure) 6. FSH, TSH, PRL 7. If absent menses, do progestin challenge 8. If dysmenorrhea, do free testosterone Hysterosalpingography +/- hysteroscopy Laparoscopy Semen analysis |
|
|
When is the optimal time for coitus in order to maximize chances of conception?
|
0-2 days before ovulation
|
|
|
Diagnostic criteria for polycystic ovarian syndrome.
|
2 of 3 to make diagnosis
1. oligomenorrhea/irregular menses for 6 months 2. clinical or lab evidence of hyperandrogenism 3. polycystic ovaries on U/S |
|
|
Pathogenesis of PCOS?
|
Not well understood.
Starts with increased GnRH pulsatility, which for some reason causes ↑LH, ↓FSH. The ↑LH causes theca cells to produce more androgen. ↑androgen inhibits follicular development. In addition, there is ↑insulin, due to insulin resistance. - Insulin works with LH to increase theca cell androgen production. - Also decreases SHBG, which increases free T. |
|
|
Treatment of PCOS.
|
Weight loss.
Metformin. If not trying to conceive, use oral contraceptives, because chronic unopposed estrogen can cause endometrial cancer. If trying to conceive, clomiphene citrate (for 5 days) to stimulate ovulation. |
|
|
What are estrogen levels like in women with PCOS?
|
It is usually normal. The granulosa cells are still able to convert androgen to estrogen. However, they don't have surges of estrogen, because their follicles don't mature (due to excess androgen)
|
|
|
Which ovarian hormone causes Spinnbarkeit?
|
Spinnbarkeit = stretchy stringy quality of cervical mucus just prior to ovulation. This is the time when sperm is able to penetrate the mucus.
Estrogen causes it. |
|
|
What is simplest and most accurate test to confirm ovulation?
|
Mid-luteal serum progesterone assay.
|
|
|
Which phase of ovarian cycle is fixed at 2 weeks?
|
Luteal phase is fixed at 14 days.
Follicular phase is variable. |
|
|
What happens to basal body temperature during the ovarian cycle? What causes the change?
|
Progesterone causes a 0.5-1˚ rise in temperature during luteal phase
|
|
|
What is the ratio of LH:FSH that you'd expect in PCOS?
|
Greater than or equal to 3:1
|
|
|
How does clomiphene citrate work?
|
It is a SERM, and blocks estrogen receptors at the hypothalamus. The hypothalamus is tricked into thinking that estrogen levels are low, and produces more GnRH. This causes increased LH and FSH, which results in ovulation.
|
|
|
Which hormone produced by corpus luteum inhibits ovulation?
|
Progesterone
|
|
|
Definition of amenorrhea.
|
1. No period by 14 year old, absence of growth or development of 2° sexual characteristics
2. No period by 16 year old, regardless of presence of normal growth and development or the appearance of 2° sexual characteristics 3. No period for length of at least 3 previous cycle intervals, or no periods for 6 months |
|
|
Define oligomenorrhea, polymenorrhea, metrorrhagia.
|
• Oligomenorrhea - intervals > 35 days
• Polymenorrhea - intervals < 21 days • Metrorrhagia - irregular intervals with excess flow and duration |
|
|
Approach to amenorrhea.
|
Hypogonadal (↓estrogen)
• Hypergonadotropic (↑LH/FSH) - Savage syndrome (mutated FSH receptor) - Swyer syndrome (mutated/deleted SRY) - Primary ovarian failure, could be APS-II • Hypogonadotropic (↓LH/FSH) - Anorexia, stress, excess exercise - Prolactinoma - Hypothyroidism - Craniopharyngioma - Sheehan syndrome - Kallmann syndrome (GnRH deficiency + anosmia) - Idiopathic hypogonadotropic hypogonadism Eugonadal (Normal estrogen) • Chronic anovulation (PCOS) • Outflow tract abnormality - Asherman syndrome (intrauterine adhesions) - Transverse vaginal septum - Imperforated hymen - Müllerian agenesis - Androgen insensitive syndrome |
|
|
What is Kallmann syndrome?
|
A cause of amenorrhea.
Caused by congenital GnRH neuron migration disruption, causing isolated GnRH deficiency. Also associated with anosmia, sometimes with midline facial defects. |
|
|
What is Swyer syndrome?
|
It is 46, XY, with SRY mutation or deletion. Looks female, but do not have functioning gonads. Causes hypergonadotropic hypogonadism.
A cause of amenorrhea. |
|
|
What is Savage syndrome?
|
Autosomal recessive. Mutation in FSH receptor, causing FSH insensitivity. Causes hypergonadotropic hypogonadism.
A cause of amenorrhea. |
|
|
What is dysfunctional uterine bleeding?
What is the treatment? |
Anovulatory irregular bleeding.
- PCOS - Postmenarcheal women - Perimenopausal women - Hypo/hypo (they have low estrogen, and so vessels are close to the surface, break easily) Treatment: - For amenorrheic anovulatory women, give cyclic protestin, 10 mg x 10-14 days. - For women who ovulate occasionally, give OCP to over-ride natural system. |
|
|
What is the disadvantage to giving continuous combined OCP?
|
They have progesterone >>> estrogen. So chronic use will cause thinning of endometrial lining, and subsequent spotting. May want to encourage them to use cyclic schedule - 3 weeks on, 1 week off.
|
|
|
Define natural menopause.
Define early menopause. Define perimenopause. |
Natural menopause = 12 consecutive months of amenorrhea, caused by permanent loss of ovarian follicular activity.
Early menopause = when it occurs before age 40. Perimenopause = period of time prior to menopause, and for the first year after menopause. |
|
|
What are non-hormonal treatments for vasomotor symptoms from menopause?
|
Vitamin E
SSRI Gabapentin Clonidine |
|
|
How does menopause cause depression?
|
Estrogen has positive effect on serotonin activity.
□ Up-regulation of 5-HT₁ receptors □ Decreases monoamine oxidase (MAO) activity |
|
|
What are important changes in a postmenopausal woman?
|
1. Urogenital concerns (UTI, incontinence, atrophy, pelvic prolapse)
2. Sexual concerns 3. Depression 4. Osteoporosis 5. Cardiovascular disease (lipids, DM, thrombosis) |
|
|
What is used to prevent osteoporosis in postmenopausal women?
|
Bisphosphonates or raloxifene.
Do not use hormonal therapy, unless there is significant risk of osteoporosis that outweighs the risks of HT. |
|
|
Is there increased or decreased risk of ovarian cancer with HT?
|
There may be increased risk with the use of estrogen only HT over long term. Not proven yet.
|
|
|
Does HT help protect against cardiovascular disease?
|
Only if started early menopause (<65 years old).
If it is started later, it increases CAD, because it induces MMP-9, which destabilizes plaque in arterial walls. |
|
|
Risks and benefits of EPT over estrogen-only therapy.
Why choose one over the other? |
Estrogen + progesterone therapy - use for women who have not had hysterectomy
• Risks • VTE • Stroke • Breast cancer (only after 5+ years of HT use) • Benefits • Quality of life • Bone density • Colon cancer Estrogen-only therapy - use for women that have had hysterectomy • Risks • VTE • Stroke • Benefits • Quality of life • Bone density |
|
|
What are indications for HT use?
|
1. Moderate to severe vasomotor symptoms.
2. Topical use for vulvar/vaginal atrophy. 3. Premature ovarian failure, until age 51. |
|
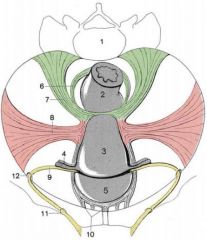
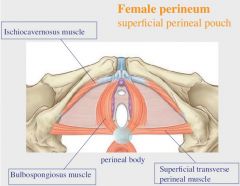
Name the 4 fibromuscular ligaments of the uterus.
|
6 = rectouterine ligament
7 = sacrouterine ligament 8 = cardinal ligament 9 = round ligament |
|
|
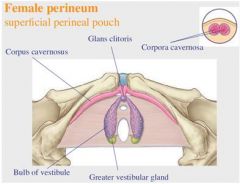
|
|
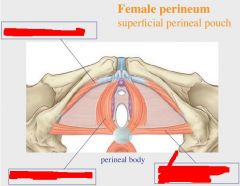
|
|
|
|
What is the path of the pudendal nerve?
|
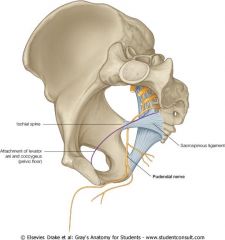
Exits through greater sciatic foramen, loops around sacrospinous ligament, and re-enters through lesser sciatic foramen
|
|
|
What are the three branches of the pudendal nerve?
|
Dorsal nerve of clitoris/penis
Perineal nerve Inferior rectal nerve |
|
|
What are symptoms of hypoestrogenism?
|
• Insomnia
• Lack of energy • Urinary frequency • Superficial dyspareunia • Hot flushes Remember - NOT nausea, as hyperestrogenism is what causes nausea. |
|
|
Contrast the side effects of estrogen therapy vs. progesterone therapy.
|
They both cause breast tenderness and fluid retention.
E causes nipple sensitivity and vaginal discharge. P causes bloating and increased appetite. |
|
|
What is the pH of the vagina in reproductive years? in postmenopausal years?
|
<4.5
6-7.5 |
|
|
What is the minimal lab tests that must be ordered for investigating secondary amenorrhea?
|
Pregnancy test
FSH - r/o ovarian failure TSH - r/o thyroid disease Prolactin - r/o hyperprolactinemia Progestin challenge test will help determine estrogen status If there are signs of hyperandrogenism, also check DHEA-S or testosterone. |
|
|
If a secondary amenorrheic woman is given a progestin challenge test and she has a withdrawal bleed, what does this mean?
What would be the follow up test if she did not have withdrawal bleed? |
It means that she had normal, unopposed estrogen levels before, and so the likely diagnosis is that she has PCOS.
If she did not bleed, then the next test would be to do an E+P cycle. If she bled from that, the dx would be either POF or hypothalamic hypogonadism. If she did not bleed from that, the dx would be endometrial scarring, such as Asherman syndrome. |
|
|
How is the diffusion gradient for CO₂ maintained between the maternal and fetal blood?
|
Maternal hyperventilation.
|
|
|
What is the function of estrogen in process of parturition?
|
□ Gap junctions in myometrium
□ Oxytocin receptors upregulation in third trimester □ Prostaglandin synthesis in membranes and decidua |
|
|
When is hCG first present in maternal blood? When can it be detected in a urine test? When do levels reach peak?
|
8-9 days post-ovulation.
Can be detected in urine 2-4 days before missed period. Levels double every 48 hours, until 10 weeks, and then slowly decreases |
|
|
What is the function of hCG?
|
- Maintaining corpus luteum and the progesterone production which is essential to early pregnancy development
- Gonadotropic role on fetal testis |
|
|
How much of the somatotropic activity and prolactin activity does hPL have?
|
- 3% the activity of GH, 50% the activity of PRL
|
|
|
How does the coomposition of fetal amniotic fluid change with increasing gestational age?
|
Starts off isotonic with maternal plasma. Then, with increasing age...
- Decreasing osmolality - Decreasing sodium - Increasing urea - Increasing creatinine - Increasing uric acid - Increasing hair, desquamated fetal cells, vernix caseosa - Increasing glycerophospholipids from lungs |
|
|
How much fetal lung fluid is produced per day at term?
|
400 cc. Of this amount, 200 cc is swallowed, and 200 cc is excreted in amniotic fluid.
|
|
|
How is amniotic fluid resorbed by the fetus?
|
Swallowing starts in week 16. About 800 cc/day.
Intramembranous flow (resorption through placenta). About 200 cc/day. |
|
|
What are causes of oligohydramnios? Polyhydramnios?
|
Decrease in amniotic fluid level (oligohydramnios)
• ↓production secondary to renal anomaly • ↓production secondary to fetal stress and hypoxia Increase in amniotic fluid level (polyhydramnios) • ↑production secondary to - Increased maternal blood sugar - Increased urine output of fetus • ↑production at placental surface secondary to placental anomaly • ↓swallowing from anencephaly or esophageal atresia |
|
|
What is pre-eclampsia?
|
Hypertension with proteinurea or edema during pregnancy.
|
|
|
What happens to pH and pCO₂ and HCO₃⁻ if fetus is hypoxic for a short time? long time?
|
Short time - respiratory acidosis. Low pH, high pO₂.
Long time - metabolic acidosis. Low pH, low HCO₃⁻. |
|
|
What does it mean if the base excess/deficit is high?
|
The larger the absolute number, the more base has been depleted. The worse the severity/duration of impaired fetal oxygenation.
Normal is -5.5 to 0. |
|
|
What are uterotropins and uterotonins?
What counteracts uterotropins? |
Uterotropins - set stage for contractions
- Ca2+ - Arachidonic acid - Relaxin - Estrogen Counteracted by progesterone Uterotonins - actually cause contraction - Endothelin-1 - Oxytocin - Prostaglandin |
|
|
Which hormone is important in maintaining uterine quiescence in pregnancy?
|
Progesterone, by opposing uterotropins, decreasing myometrial gap junctions, downregulating oxytocin receptors on myometrium, decreasing prostaglandin production.
|
|
|
What is the difference between postpartum blues and postpartum depression?
|
Blues
- 80% of women - teary - < 2 weeks - no treatment Depression - 10-25% of women - a medical condition that requires treatment |
|
|
What is Chadwick sign?
|
At 8-12 weeks GA, bluish tinge to vulva, vagina and cervical tissue, due to increased blood flow causing venous congestion.
|
|
|
What is it called when the superficial layer of the decidua is sloughed following delivery?
|
Lochia rubra (red) - first few days
Lochia serosa (pinkish brown) - after 4 days Lochia alba (yellow white) - after 10 days |
|
|
What is Hegar sign?
|
At 6 weeks GA, the lower uterine segment softens and is easily palpable.
|
|
|
Blood flow increases with gestational age to all maternal organs except the...
|
Liver
|
|
|
What features or associated signs/symptoms of an adnexal mass would make you suspicious that it is pathological, and not benign?
|
> 6-8 cm in size
Complex (both solid and cystic) Bilateral Solid Ascites If the woman is premenarchal, or postmenopausal |
|
|
What is it called when a woman has a benign ovarian tumour, ascites, and pleural effusion?
|
Meigs syndrome
|
|
|
What is a Krukenberg tumor?
|
An ovarian tumor that is metastatic from the GI tract.
|
|
|
Staging of ovarian cancer
|
Stage I: confined to ovary
□ Ia - one ovary □ Ib - both ovaries □ Ic - rupture or tumour on surface Stage II: confined to pelvis Stage III: confined to abdomen Stage IV: distant disease |
|
|
What genes confer an ovarian cancer risk?
|
BRCA1 and BRCA2 account for 90% of hereditary ovarian cancers
|
|
|
Contrast partial and complete hydatidiform mole
|
Partial mole
- 69 XXY - fetus often present - small uterus for date - theca lutein cysts rare - medical complications low - malignancy potential low Complete mole - 46 XX, but all from paternal genome - fetus absent - large for uterus date - theca lutein cysts often - medical complications frequent - malignant potential high (20%) |
|
|
What are the factors that increase risk of hydatidiform mole?
|
Prior molar pregnancy
Prior spontaneous abortion Maternal age > 35 Vitamin A deficiency |
|
|
After a dilation and curettage treatment of hydatidiform mole, what follow-up tasks are necessary?
|
Chest x-ray and LFTs - look for metastases.
Check β-hCG weekly until normal, and then monthly for 12 months. Contraception x 12 months, because if they get pregnant, don't know if it is a relapse or pregnancy. |
|
|
What are the types of malignant gestational trophoblastic neoplasias that occur after a molar pregnancy?
|
1. Invasive mole
2. Choriocarcinoma 3. Placental site trophoblastic tumour Hard to tell difference between 1 and 2. Need to look at tumour markers. For PSTT, hPL will be elevated. Chemoresistant, so need hysterectomy. |
|
|
What are the most common sites of metastases for malignant gestational trophoblastic neoplasias?
|
Lung
Pelvis Vagina Brain Liver |
|
|
What is the treatment for malignant GTN?
|
Low risk (based on prognostic factors): single agent chemo (methotrexate or actinomycin D)
High risk: combination chemo There is limited role for surgery, except for PSTT (placental site trophoblastic tumour), which requires hysterectomy. |
|
|
Which gynecological cancers cannot/aren't treated with radiation therapy?
|
Vulva/lower vaginal - causes severe sloughing.
Ovarian/fallopian tubes - RT does not work. |
|
|
What genetic disease causes increased risk of uterine cancer?
|
Lynch syndrome (HNPCC)
|
|
|
What is the best diagnostic test for endometrial cancer?
|
Endometrial aspiration biopsy.
|
|
|
Name the different pathological types of endometrial cancer.
|
• Endometrioid (most common - 75-80% of cases)
• Mucinous - 5% • Serous - < 10% • Clear-cell - 4% • Squamous, mixed, metastatic |
|
|
Staging of endometrial cancer. How is it staged, and what are the stages?
|
Surgically (TAH-BSO, with pelvic and para-aortic lymph node dissection)
Stage I - no invasion, or only invades myometrium Stage II - invades endocervical stroma Stage III - local/regional spread □ A - adnexa and/or serosa □ B - vaginal/parametrial metastases □ C - lymph nodes (pelvic or para-aortic) Stage IV - bowel/bladder or distant metastases |
|
|
Which strains of HPV cause high grade squamous intraepithelial lesions (HSIL) of cervix?
Which strains cause LSIL and warts? |
HSIL: 16, 18.
LSIL and warts: 6, 11. |
|
|
Which lymph nodes does cervical cancer first spread to?
|
Pelvic and para-aortic lymph nodes
|
|
|
What are risk factors for developing cervical cancer?
|
HPV (sex)
Smoking Immunosuppression |
|
|
When can a woman stop pap smears?
|
Age 69, if 3 or more normal smears in the last 10 years, and no history of previous significant abnormalities.
|
|
|
What are the different stages of cervical cancer? (stage 1-4)
|
Stage I - confined to cervix.
Stage II - extend beyond cervix, but does not extend to pelvic wall. Does not reach lower 1/3 of vagina. Stage III - extends to pelvic wall, and no space is felt between the tumour and pelvic wall. Tumour involves lower 1/3 of the vagina. Stage IV - extends beyond true pelvis, and involves bladder or rectum. |
|
|
What are the possible results from a pap smear?
|
Normal
Atypical squamous cells - ASC-US (undetermined significance) - ASC-H (can't exclude HSIL) LSIL (low grade squamous intraepithelial lesion) - a transient HPV infection, usually corresponds to CIN-1 HSIL (high grade SIL) - persistent HPV infection, higher risk of cancer. Corresponds to CIN-2, 3, or CIS. Can also see glandular cells, which would indicate endometrial cancer. |
|
|
What are the subtypes of ovarian carcinoma, from most common to least common?
|
1. High-grade serous carcinoma (70%)
2. Clear cell ca (10%) 3. Endometrioid ca (10%) 4. Low-grade serous ca (<5%) 5. Mucinous ca (<5%) |
|
|
What are risk factors for endometrial cancer?
|
Obesity
Hypertension Diabetes Exogenous estrogens Estrogen secreting ovarian tumours PCOS (unopposed estrogen) |
|
|
What is the most common symptom of cervical cancer?
|
Post-coital bleeding
|
|
|
What is the most common symptom of molar pregnancy?
|
Abnormal vaginal bleeding
|
|
|
What is the most common symptom of endometrial hyperplasia?
|
Vaginal bleeding
|
|
|
What is the most predictive serum marker for epithelial adenocarcinoma of the ovary?
|
CA-125
|
|
|
What is the normal amount of blood lost during a menses?
|
25-75 ml
|
|
|
What are some alternatives to HT for hot flashes due to menopause?
|
Venlafaxine
Clonidine Gabapentin |
|
|
What are the most common causes of vaginal bleeding in the 3rd trimester?
|
Placenta previa (not painful)
Abruptio placenta (painful) |

