![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
64 Cards in this Set
- Front
- Back
|
What substance has the most effect on the vascular tone of the afferent arterioles in the kidney? Efferent?
|
Afferent: PGE2 (renal-derived) causes vasodilation.
Efferent: Angiotensin II causes vasoconstriction of the efferent arterioles (increasing GFR). |
|
|
What enzyme in the kidney activates vitamin D? What are the functions of vitamin D?
|
1-alpha-hydroxylase is synthesized in the proximal renal tubule cells and converts 25-hydroxycholecalciferol to 1,25-dihydroxy...
Functions: increase GI reabsorption of Ca and PO4; promote bone mineralization (stimulates ALP release from bone, ALP inhibits pyrophosphate which normally inhibits crystallization); increases production of osteoclasts from macrophage stem cells. |
|
|
What are the most common upper urinary tract causes of hematuria? Lower?
What drugs are associated with hematuria? |
Upper: renal stones, glomerulonephritis, renal cell carcinoma.
Lower: infection, TCC (most common cause of gross hematuria in the absence of infection), BPH. Drugs: anticoagulants (1) cyclophosphamide. |
|
|
What qualitative urinary test do you use to measure the amount of albumin in urine? Albumin and globulins?
|
Dipsticks are specific for albumin. Sulfosalicylic acid detects albumin and globulins. Qualitative test is a 24-hour urine collection.
Persistent proteinuria: usually indicates intrinsic renal disease. |
|
|
What are the causes of increased serum BUN?
|
Decreased CO (decreases GFR which increases reabsorption of urea).
High-protein diet/blood in GI. Increased catabolism: third degree burns. Acute glomerulonephritis (decreases GFR). Renal failure: ATN, diabetic glomerulopathy. Postrenal disease: stone, BPH (decreases GFR). |
|
|
What affect does SIADH have on serum BUN?
|
SIADH increases plasma volume thus increasing GFR and decreasing serum BUN. Other causes of decreased serum BUN: pregnancy (same mech.); decreased urea synthesis (liver disease); decreased intake (Kwashiorkor).
|
|
|
Name the four types of proteinuria? Explain the type that is not associated with renal disease.
|
Functional (<2g/24hrs): not associated with renal disease. Causes: fever, exercise, CHF. Orthostatic: occurs with standing and is absent lying down; urine protein is absent in the morning.
Other three: overflow, glomerular, tubular. |
|
|
What causes the following types of proteinuria:
1) Overflow 2) Glomerular 3) Tubular |
1) Amount filtered > tubular reabsorption of proteins. Multiple myeloma (BJ), hemoglobinuria, myoglobinuria (crush injury, McArdles).
2) Nephritic syndrome (damaged GBM and loss of albumin and globulins). Nephrotic syndrome (loss of negative charge on GBM and loss of albumin). 3) Defect in proximal tubule reabsorption. Heavy metals, Fanconi's, Hartnup's. |
|
|
What causes an increase serum BUN:Cr ratio (> 15)?
|
Azotemia: increase in serum BUN and creatinine. Causes of increase BUN:Cr (both have no intrinsic kidney damage):
Prerenal azotemia: decreased cardiac output (decreases GFR, but some urea is reabsorbed back into the blood). Postrenal azotemia: acute urinary tract obstruction. |
|
|
What pathologies result in a normal to decrease in serum BUN:Cr ratio (< 15)?
|
Azotemia: increase in serum BUN and creatinine. BUN:Cr ratio increases in renal azotemia (uremia) caused by parenchymal damage to the kidneys (e.g. acute tubular necrosis, chronic renal failure).
|
|
|
What are the commonest causes of increased creatinine clearance? Decreased CCr?
|
Increased: normal pregnancy (1st trimester, due to increase in plasma volume), early diabetic glomerulopathy (efferent arteriole hyaline arteriolosclerosis).
Decreased: elderly (annual decrease in CCr of 1 mL/min beyond age 50), acute/chronic renal disease. |
|
|
What causes an increase in serum glucose and glucosuria?
What causes normal serum glucose and glucosuria? |
1) Diabetes mellitus
2) Normal pregnancy, benign glucosuria: both conditions have decreased renal threshold for glucose. |
|
|
Name some causes of ketonuria.
|
Diabetic ketoacidosis, starvation, ketogenic diets, pregnancy. Test only detects acetone and acetoacetone (not beta-hydroxybutyrate).
|
|
|
In what pathologies would you see the following on a urinalysis:
1) bilirubinuria 2) absent UBG 3) increased UBG 4) hemoglobinuria |
1) Viral hepatitis, obstructive jaundice.
2) Obstructive jaundice. 3) Viral hepatitis, extravascular hemolytic enemia. 4) intravascular hemolytic anemia. |
|
|
In what pathologies would you see the following on a urinalysis:
1) Myoglobinuria 2) Increased nitrites 3) Leukocyte esterase |
1) Crush injuries.
2) Nitrate-reducing uropathogens (E. coli). 3) Presence of esterase in neutrophils (pyuria) - infections (urethritis, cystitis, pyelonephritis); sterile pyuria - chlamydia trachomatis urethritis, renal tuberculosis. |
|
|
What casts can you find in sediment urinalysis?
|
Hyaline - no significance in absence of proteinuria (normal individual after exercise or dehydration).
RBC - nephritic glomerulonephritis. WBC - acute pyelonephritis, acute tubulointerstitial nephritis. Renal tubular cell - ATN. Fatty - nephrotic syndrome. Waxy - chronic renal failure. Broad - chronic renal failure (waxy cast with large diameter). |
|
|
What crystals can be found in urinalysis?
|
Calcium oxalate, uric acid, cystine.
|
|
|
What part of the glomerulus is negatively charged? What molecules are permeable?
|
The glomerular basement membrane composed of type IV collagen is negatively charged (heparan sulfate). Cationic proteins of LMW are permeable, as well as water and LMW proteins (e.g. AA's).
|
|
|
What produces the glomerular basement membrane?
|
Visceral epithelial cells, which contain podocytes and slit pores (between the podocytes).
|
|
|
When are podocytes fused?
|
Fusion of the podocytes is present in any cause of the nephrotic syndrome.
|
|
|
Why are mesengial cells within the glomerulus significant in some pathological disorders?
|
Mesangial cells normal support the glomerular capillaries. They can also release inflammatory mediators and proliferate (e.g. IgA glomerulonephritis).
|
|
|
What is the most common congenital kidney disorder?
|
Horseshoe kidney (majority are fused at the lower pole, 90%). Increased incidence with Turner's syndrome. Danger of infection and stone formation.
|
|
|
Young child has an enlarged, irregular, cystic, unilateral flank mass. Further investigation reveals abnormal cartilage in the kidney. Play odds, what does the child have?
|
Renal dysplasia - most common cystic disease in children. No inheritance.
|
|
|
This child kidney disease leads to Potter's syndrome. What is the disease and what is features are characteristic of Potter's?
|
Potter's is due to maternal oligohydramnios - low-set ears, parrot beak nose, and lung hypoplasia. The autosomal recessive disorder, Juvenile polycystic kidney disease, can lead to bilateral cysts in the cortex and medulla. Cysts also occur in the liver - associated with hepatic fibrosis leading to portal HTN.
|
|
|
A 50 year old lady presents to you office with a plethora of problems. She has bilateral palpable kidneys, hypertension, and hematuria. After determining she has an autosomal dominant disorder, uou decide to run further test, suspecting she may have sigmoid diverticulosis, mitral valve prolapse or even renal cell carcinoma.
|
Adult polycystic disease: develops by 20-25 and chronic renal failure begins at 40-60 years of age. Cysts are present in the liver (40%) and pancreas. HTN (80%), berry aneurysms (10-30%).
|
|
|
Your patient has recurrent UTI's, hematuria, and renal stones. The papillary ducts of the medulla have a Swiss cheese appearance. Whats the diagnosis?
|
Medullary sponge kidney.
|
|
|
What is the most common cause of acquired polycystic kidney disease?
|
Renal dialysis (50% of patients on long term dialysis). Tubules are obstructed by interstitial fibrosis or oxalate crystals. Small risk for developing renal cell carcinoma.
|
|
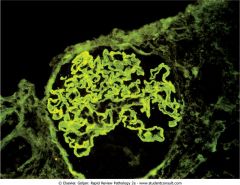
Name the pathology.
|
Linear pattern on inmmunofluorescence stain. Characteristic finding in anti-GBM disease (Goodpasture syndrome).
|
|
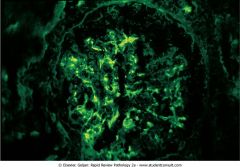
Name the pathology of the kidney.
|
Immunofluorescence stain of the kidney showing granular immunofluorescence. Granular irregular deposits in the capillaries are caused by immunocomplex deposition (postreptococcal glomerulonephritis).
|
|
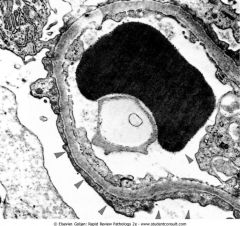
What does this EM of the kidney show?
|
Fusion of the podocytes, which should be separated by slit pores. This finding occurs in all glomerular diseases that present with the nephrotic syndrome (e.g. minimal change disease).
|
|
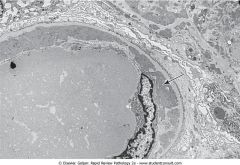
What does this EM of the kidney show?
|
Subendothelial immunocomplex deposits directly beneath the nucleus of the endothelial cell. A thin rim of normal BM separates the deposits from the epithelial side of the membrane. The patient had diffuse proliferative glomerulonephritis due to SLE.
|
|
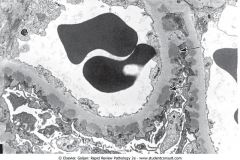
What does this EM of the kidney show?
|
Subepithelial immunocomplex deposits directly beneath the visceral epithelial cells in a patient with poststreptococcal glomerulonephritis.
|
|
|
Explain the damage done to kidneys in SLE.
|
DNA-anti-DNA complexes in SLE deposit in glomeruli where they activate complement system. C5a is produced which is chemotactic to neutrophils. Neutrophils damage the glomeruli.
|
|
|
Compare nephritic and nephrotic.
|
Nephritic: RBC casts, glomerular inflammation, proteinuria < 3.5 g/24 hrs., oliguria, HTN more often.
Nephrotic: proteinuria > 3.5 g/24 hrs., fatty cast, pitting edema. |
|
|
What are the clinical and laboratory findings in nephritic glomerular syndrome?
|
HTN - due to salt retention.
Periorbital puffiness - salt retention. Oliguria - inflammed glomeruli decreases GFR. Hematuria - dysmorphic RBCs with irregular membranes. Neutrophils in the sediment. RBC casts. Proteinuria < 3.5 g/24hrs. Azotemia with BUN:Cr > 15. |
|
|
How would IgA glomerulopathy present clinically?
|
Most common GN; nephritic or nephrotic presentation. The patient (child or adult) would have episodic bouts of hematuria, usually following an upper respiratory infection. Mesangial IgA IC deposits with granular IF.
|
|
|
Is SLE a nephritic or nephrotic disease?
|
Most often a primary nephritic. Subendothelial IC depositis with granular IF; DNA-anti-DNA activate complement. Diffuse proliferative GN. Most common cause of death in SLE patients is CRF.
|
|
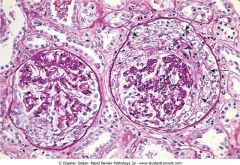
Proliferation of parietal epithelial cells in Bowman's capsule. What is this?
|
Crescentic glomerulonephritis Clinically associated with Goodpasture, microscopic polyarteritis, Wegener's granulomatosis.
|
|
|
What are the clinical and laboratory findings in nephrotic syndrome?
|
Proteinuria > 3.5 g/24hrs.
Pitting edema/ascites - due to hypoalbuminemia. Risk for developing spontaneous peritonitis. HTN (more common in nephritic). Hypercoagulable state - loss of ATIII. Hypercholesterolemia - hypoalbuminemia increases cholesterol (unknown). Hypogammaglobulinemia. fatty casts. |
|
|
This nephrotic syndrome is more common in girls than boys. T-cell cytokines cause the GBM to lose its negative charge resulting in selective proteinuria (albumin not globulins). It is often preceded by respiratory infection or immunization. What histological changes are seen in this syndrome?
|
Minimal change disease (lipoid nephrosis) has positive fat stains in glomerulus and tubules. Fusion of podocytes are present. Children respond well to corticosteroids.
|
|
|
What glomerulonephritis is most common in HIV patients and IV heroin abuse?
|
Focal segmental glomerulosclerosis. Focal damage of visceral epithelial cells. Nonselective proteinuria, microscopic hematuria. Progresses to chronic renal failure.
|
|
|
What is the most come nephrotic syndrome in adults and what precedes the onset?
|
Diffuse membranous glomerulopathy. Drugs (e.g. captopril), infections (HBV, Plasmodium, syphilis), malignancy (NHL), autoimmune.
|
|
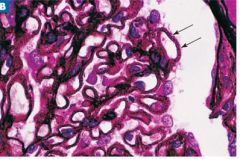
Silver stain of the glomeruli. Diagnosis?
|
Diffuse membranous glomerulonephritis. Silver stain shows numerous silver-positive spikes on the epithelial side of the GBM (subepithelial IC deposits).
|
|
|
Explain the pathogenesis of MPGN type I and II.
|
MPGN - Subendothelial IC with granular IF. IC activate compliment. Associated with HBV, HCV, or cryoglobulinemia.
MPGN II - C3 nephritic factor (C3NeF) is an autoantibody that binds to C3 convertase preventing its degredation and causing sustained activation of C3 (therefore, low C3 levels). |
|
|
What are the risk factors for diabetic patients developing diabetic glomerulopathy?
|
Type 1 > type 2. Poor glycemic control, HTN, diabetic retinopathy (high correlation with coexisting glomerulopathy).
|
|
|
What is the pathogenesis of diabetic glomerulopathy?
|
Nonenzymatic glycosylation of the GBM leads to hyaline arteriolosclerosis (afferent before efferent).
Osmotic damage to glomerular capillary endothelial cells (sorbitol). Afferent arteriolosclerosis leads to hyperfiltration damage to the mesangium. Diabetic microangiopathy - increased deposition of type IV collagen. Fusion of podocytes. |
|
|
What is the initial laboratory manifestation of diabetic glomerulopathy?
|
Microalbuminuria after ~ 10 years of poor glycemic control.
|
|
|
What hereditary glomerular disease may you find lipid accumulation in visceral epithelial cells (foam cells)?
|
Alport's syndrome. X-linked dominant. Defect in the synthesis of type IV collagen in GBM.
|
|
|
Name some causes of acute renal failure. (ARF is suppression of renal function developing in 24 hrs, accompanied by anuria or oliguria).
|
Most common: acute tubular necrosis (ischemic or nephrotoxic).
Postrenal obstruction (e.g. BPH, invasive cervical cancer). Vascular disease (malignant HTN). Rapid progressive GN. DIC, urate nephropathy. |
|
|
What is the consequence of ischemic ATN?
|
Ischemia damages endothelial cells causing a release in endothelin and vc of afferent afterioles.
Ischemia damages tubule cells. Detachment of tubular cells into the lumen causing obstruction produces pigmented renal tubular cell casts. Casts obstruct lumen leading to decreased GFR, pushes fluid into interstitium, oliguria. |
|
|
What sites of the kidney are most susceptible to ischemic ATN?
|
Straight segment of the proximal tubule and medullary segment of the thick ascending limb.
|
|
|
What is the most common cause of nephrotoxic ATN?
|
Aminoglycosides (e.g. gentamicin).
|
|
|
Why does nephrotoxic ATN have a better prognosis than ischemic ATN?
|
The tubular basement membrane is left intact in nephrotoxic.
Ischemic: prerenal azotemia (decrease CO). Nephrotoxic: aminoglycosides, radiocontrast agents, heavy metals. |
|
|
Tubulointerstitial nephritis (TIN) is acute or chronic inflammation of tubules and interstitium. There are many causes including drugs, infections (Legionnaires, leptospirosis) SLE, urate nephropathy, multiple myeloma. What is the most common cause?
|
Acute pyelonephritis.
|
|
|
What is acute pyelonephritis?
|
Most common cause of tubulointerstitial nephritis. W > M. RF: UT obstruction, medullary sponge kidney, DM, pregnancy, HbSS.
Pathogenesis: vesicoureteral reflux with ascending infection. |
|
|
Women with HbAS presents with spiking fever, flank pain, increased frequency of urination and dysuria. Urinalysis shows WBC casts, E.coli, and hematuria. What complications could arise from this syndrome?
|
Tubulointersitial nephritis. Complications: chronic pyelonephritis, perinephric abscess, renal papillary necrosis, septicemia with endotoxic shock.
|
|
|
What are the hallmark findings in the following types of chronic pyelonephritis:
1) Reflux 2) Obstructive |
1) U-shaped cortical scars overlying a blunt calyx.
2) Uniform dilation of calyces. Microscopic findings: chronic inflammation and tubular atrophy (thyroidization). |
|
|
Abrupt onset of fever, oliguria, and rash. Two weeks ago the patient had been treated for infection. Diagnosis?
|
Acute drug-induced tubulointerstitial nephritis. Common drug associations: penicillin (methicillin), rifampin, sulfonamides, NSAIDs, diuretics. Path: combination of type I and IV hypersensitivity. Labs: BUN: Cr ratio < 15 and eosinophilia.
|
|
|
What causes analgesic nephropathy?
|
Acetaminophen: free radicals damage renal tubules in medulla.
Aspirin: inhibits renal synthesis of PGE leaving ATII unopposed - decreased blood flow. |
|
|
What complications can occur in analgesic nephropathy?
|
Renal papillary necrosis: sloughing of renal papillae (hematuria, proteinuria, and colicky flank pain).
HTN, CRF. Renal pelvic and bladder TCC. |
|
|
How can you prevent urate nephropathy in a patient being treated for disseminated cancer?
|
Allopurinol, xanthine oxidase inhibitor.
|
|
|
How does lead poisoning cause kidney damage?
|
Decreases secretion of uric acid (urate nephropathy). Direct toxic effect produces TIN.
|
|
|
What defines chronic renal failure and what are the main causes?
|
Progressive irreversible azotemia that develops over months to years. Kidneys cannot sustain life. GRF is below 10 mL/min. Causes in descending order: DM, HTN, GN (RPGN and focal segmental), cystic renal disease (renal dysplasia in children, adult PKD).
|
|
|
Patient has secondary hyperparathyroidism, osteomalacia, and osteoporosis. If the root of pathology is due to a kidney disease, what is this syndrome?
|
Renal osteodystrophy (in chronic renal failure).
Osteitis fibrosa cystica: hypovitaminosis D. Osteomalacia: decreased mineralization, fractures and bone pain. Osteoporosis: loss of organic bone matrix and minerals. Due to chronic metabolic acidosis (excess H ions buffered by bone). |

